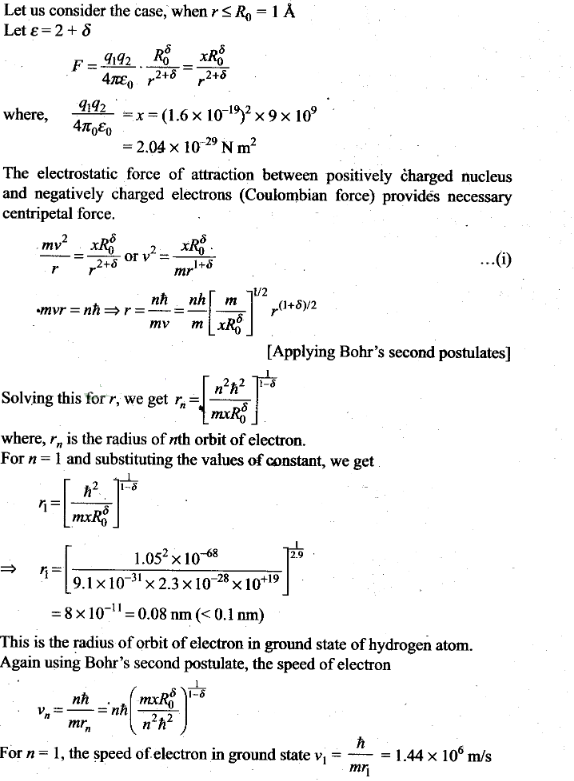
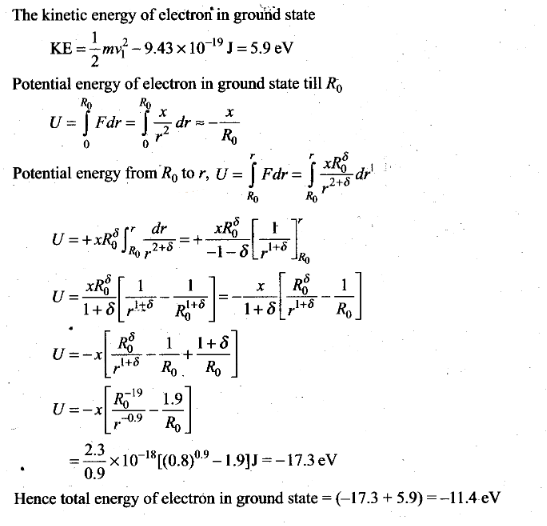
Atoms
Class 12th Physics NCERT Exemplar Solution
Multiple Choice Questions (MCQs)
Single Correct Answer Type
Question 1. Taking
the Bohr radius as a0= 53 pm, the radius of Li++ ion
in its ground state, on the basis of Bohr’s model, will be
about
(a) 53 pm (b) 27 pm (c) 18 pm
(d) 13 pm
Solution: (c)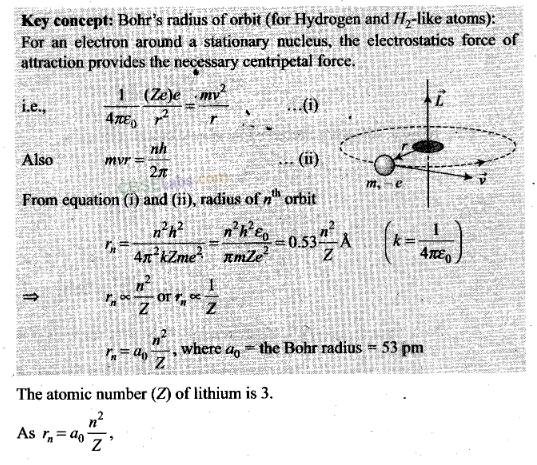

Question 2. The binding energy of a H-atom, considering an electron
moving around a fixed nuclei (proton), is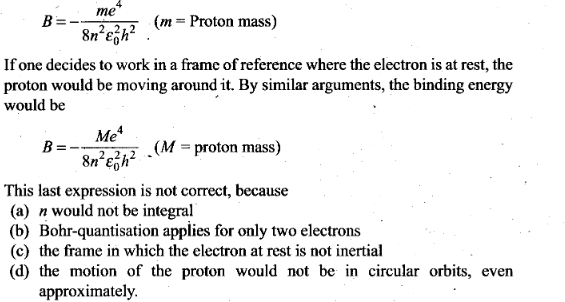
Solution: (c) In a hydrogen atom, electron
revolving around a fixed proton nucleus have some centripetal acceleration.
Therefore its frame of reference is non- inertial. If the frame of reference,
where the electron is at rest, the given expression is not true as it forms the
non-inertial frame of reference.
Question 3. The simple Bohr model cannot be directly applied to
calculate the energy levels of an atom with man electrons. This is
because
(a) of the electrons not being subject to a central
force
(b) of the electrons colliding with each
other
(c) of screening effects
(d) the
force between the nucleus and an electron will no longer be given by Coulomb’s
law
Solution: (a) The simple Bohr model cannot be
directly applied to calculate the energy levels of an atom with many electrons
because when we derive the formula for radius/energy levels etc, we make the
assumption that centripetal force is provided only by electrostatic force of
attraction by the nucleus. Hence, this will only work for single electron atoms.
In multi-electron atoms, there will also be repulsion due to other electrons.
The simple Bohr model cannot be directly applied to calculate the energy levels
of an atom with many electrons.
Question 4. For the ground state, the electron in the H-atom has an
angular .momentum = h, according to the simple Bohr model. Angular momentum is a
vector and hence there will be infinitely many orbits with the vector pointing
in all possible directions. In actuality, this is not true,
(a) because Bohr model gives incorrect values of angular
momentum
(b) because only one of these Would Have a minimum
energy
(c) angular momentum must be in the direction of
spin of electron
(d) because electrons go around only in
horizontal orbits
Solution: (a)
Bohr’s model gives only the magnitude of angular momentum.
The angular momentum is a vector quantity. Hence we cannot express angular
momentum completely by Bohr model. Hence it does not give correct values of
angular momentum of revolving electron.
Question 5. 02 molecule consists of two oxygen atoms. In the
molecule, nuclear force between the nuclei of the two atoms
(a) is not important because nuclear forces are
short-ranged
(b) is as important as electrostatic force for
binding the two atoms
(c) cancels the repulsive
electrostatic force between the nuclei
(d) is not important
because oxygen nucleus have equal number of neutrons and
protons
Solution: (a)
Key concept: Forces that
keep the nucleons bound in the nucleus are called nuclear forces.
• Nuclear
forces are short range forces. These do not exist at large distances greater
than 10~15 m.
• Nuclear forces are the strongest forces in nature.
• These
are attractive force and causes stability of the nucleus.
• These forces are
charge independent.
• Nuclear forces arc non-central force.
The nuclear
binding force has to dominate over the Coulomb repulsive force between protons
inside the nucleus. The nuclear force between two nucleons falls rapidly to zero
as their distance is more than a few femtometres.
In
O2 molecule which consists of two oxygen atoms molecules,
nuclear force between the nuclei of the two atoms is not important because
nuclear forces are short-ranged and act inside the nucleus only.
Question 6. Two H atoms in the ground state collide inelastically.
The maximum amount by which their combined kinetic energy is reduced
is
(a) 10.20 eV (b) 20.40eV (c) 13.6
eV
Solution: (a)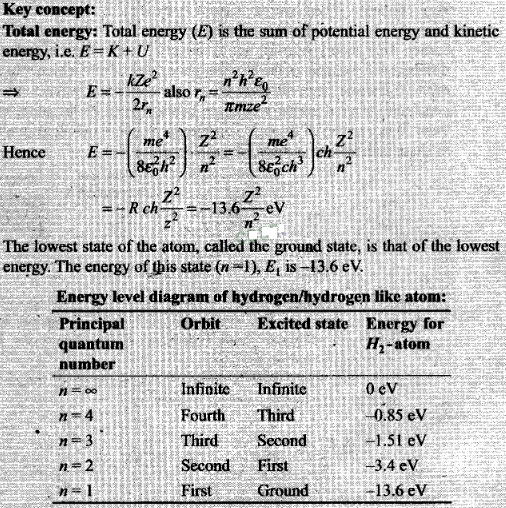
Question 7. A set of atoms in an excited state
decays
(a) in general to any of the states with lower
energy
(b) into a lower state only when excited by an
external electric field
(c) all together simultaneously
into a lower state
(d) to emit photons only when they
collide .
Solution: (a)
One or More Than One Correct Answer Type
Question 8. An ionised H-molecule consists of an electron and two
protons. The protons are separated by a small distance of the order of angstrom.
In the ground state,
(a) the electron would not move in
circular orbits
(b) the energy would be
(2)4 times that of a
H-atom
(c) the electrons, orbit would go around the
protons
(d) the molecule will soon decay in a proton and a
H-atom
Solution: (a, c) In a hydrogen atom,
electron revolves around a fixed proton nucleus in circular path. This can be
explained by Bohr model. But in case of ionised H-molecule which consists of two
protons in nucleus and where protons are separated by a small distance of the
order of angstrom, cannot be explained by Bohr model. Hence in this case the
ground state the electron would not move in circular orbits, the electrons orbit
would go around the protons.
Question 9. Consider aiming a beam of free electrons towards free
protons. When-they scatter, an electron and a proton cannot combine to produce a
H-atom.
(a) Because of energy
conservation
(b) Without simultaneously releasing energy in
the form of radiation
(c) Because of momentum
conservation
(d) Because of angular momentum
conservation
Solution: (a, b) A moving electron
and proton cannot combine to produce a H-atom because of energy conservation and
without simultaneously releasing energy in the form of radiation.
Question 10. The Bohr model for the spectra of a
H-atom
(a) will not be applicable to hydrogen in the
molecular form
(b) will not be applicable as it is for a
He-atom
(c) is valid only at room
temperature
(d) predicts continuous as well as discrete
spectral lines
Solution: (a, b) Bohr proposed
a-model for hydrogen atom which is also applicable for some lighter atoms in
which a single electron revolves around a stationary nucleus of positive charge
Ze (called hydrogen like atom, e.g.: H, He+, Li+2,
Na+1 etc). It is not applicable to hydrogen in the molecular
form and also, it will not be applicable as it is for a He-atom.
Question 11. The Balmer series for the H-atom can be
observed
(a) if we measure the frequencies of light emitted
when an excited atom falls to the ground state
(b) if
we measure the frequencies of light emitted due to transitions
between
excited states and the first excited
state
(c) in any transition in a H-atom
.
(d) as a sequence of frequencies with the higher
frequencies getting closely
Solution: (b,
d)
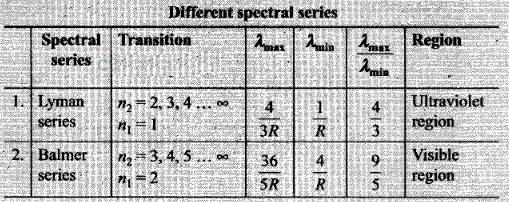
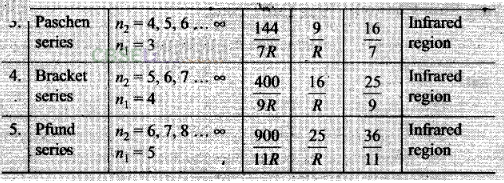
From above discussion we can say Balmer series for the H-atom
can be observed if we measure the frequencies of light emitted due to
transitions between higher excited states and the first excited state and as a
sequence of frequencies with the’higher frequencies getting closely packed.
Question 12.
Solution: (b, d) Let E2 and
E1 be the energy corresponding to n = 2 and n = 1 respectively.
If radiation of energy ∆E = (E2 – E1) = hf incident on a
sample where all the H-atoms are in the ground state, according to Bohr model
some of the atoms will move to the first excited state. As this energy is not
sufficient for transition from n = 1 to n =3, hence no atoms will make a
transition to the n = 3 state.
Question 13. The simple Bohr model is not applicable to
He4 atom because
(a) He4 is an inert
gas
(b) He4 has neutrons in the
nucleus
(c) He4 has one more
electron
(d) electrons are not subject to central
forces
Solution: (c, d)
Key concept: Bohr
proposed a model for hydrogen atom which is also applicable for some lighter
atoms in which a single electron revolves around a stationary nucleus of
positive charge Ze (called hydrogen like atom). It is valid only for one
electron atoms, e.g. : H, He+, Li+2,
Na+1 etc.
The Bohr model is not applicable to He4,
as it has one more electron and electrons are not subjected to central
forces.
Very Short Answer Type Questions
Question 14. The mass of a H-atom is less than the sum of the masses
of a proton and electron. Why is this?
Solution: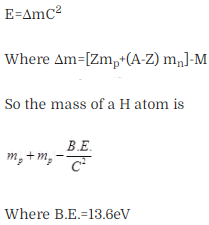
Question 15. Imagine removing one electron from He4
and He3. Their energy levels, as worked out on the basis of Bohr
model will be very close. Explain why.
Solution:
Bohr model is applicable for hydrogen atom-and some lighter atoms in which a
single electron revolves around a stationary nucleus of positive charge Ze
(called hydrogen like atom). If we remove one electron from
He4 and He3, atoms contain one electron and becomes
hydrogen like atoms. Now we can apply Bohr model to define the energy
levels.
Question 16. When an electron falls from a higher energy to a lower
energy level, the
difference in the energies appears in the
form of electromagnetic radiation. Why cannot it be emitted as other forms of
energy? .
Solution: The electrons are charged
particles. When an electron falls from a higher energy to a lower energy level,
it accelerates. We know accelerating charged particle radiates energy in the
form of electromagnetic radiation.
Question 17. Would the Bohr formula for the H-atom remain unchanged
if proton had a charge (+ 4/3)e and electron a charge (-3/4)e, where e = 1.6
x 10-19 C. Give reasons
for your answer.
Solution: According to Bohr, for
an electron around a stationary nucleus the electrostatics force of attraction
provides the necessary centripetal force.
Hence the magnitude of electrostatic force F ∞
q1 x q2
If proton had a charge (+4/3)e and
electron a charge (-3/4)e, then the Bohr formula for the H-atom remains same,
since the Bohr formula involves only the product of the charges which remain
constant for given values of charges.
Question 18. Consider two different hydrogen atoms. The electron in
each atom is in an excited state. Is it possible for the electrons to have
different energies but the same orbital angular momentum according to the Bohr
model?
Solution: Bohr postulated that an electron
in an atom can move around the nucleus in a certain circular stable orbits
without emitting radiations.
Bohr found that the magnitude of the electron’s
angular momentum is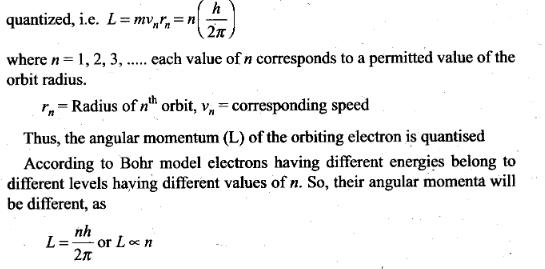
Short Answer Type Questions
Question 19. Positronium is just like a H-atom with the proton
replaced by the positively charged anti-particle of the electron (called the
positron which is as massive as the electron). What would be the ground state
energy of positronium?
Solution:
Key concept:
Positronium (Ps) is a system consisting of an electron and its anti-particle
a positron, bound together into an exotic atom, specifically anonium. The
system is unstable: the two particles annihilate each other to predominantly
produce two or three gamma-rays, depending on the relative spin states. The
orbit and energy levels of the two particles arc similar to that of the hydrogen
atom (which is a bound slate of a proton and an electron). I lowever, because of
the reduced mass, the frequencies of the spectral lines are less than half of
the corresponding hydrogen lines.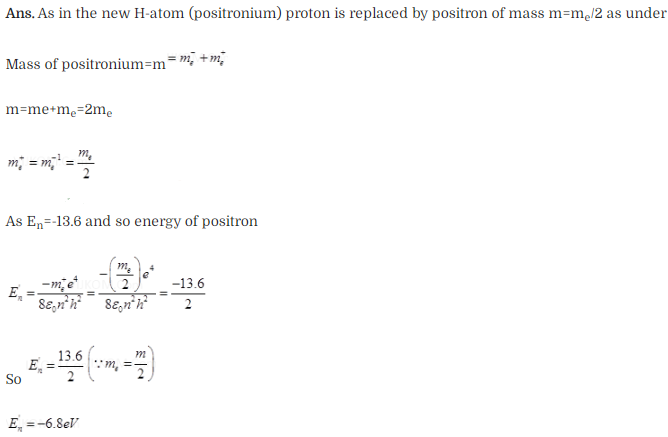
Question 20. Assume that there is no repulsive force between the
electrons in an atom but the force between positive and negative charges is
given by Coulomb’s law as usual. Under such circumstances, calculate the ground
state energy of a He-atom.
Solution: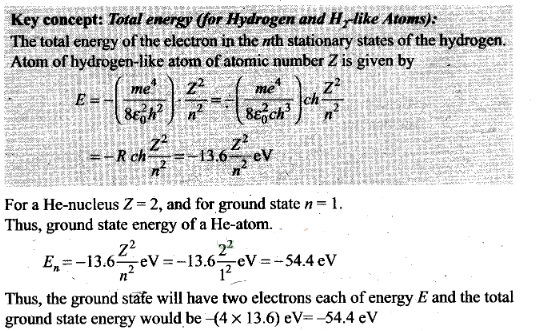
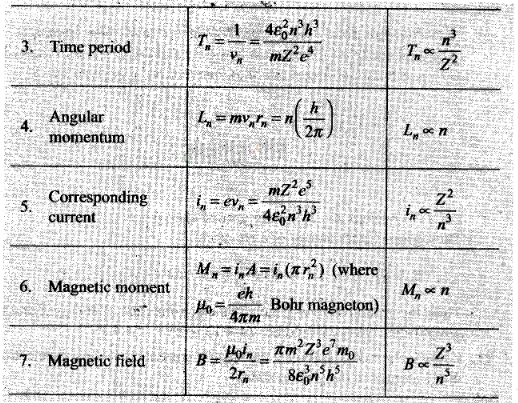
Question 21. Using Bohr model, calculate the electric current created
by the electron when the H-atom is in the ground state.
Solution: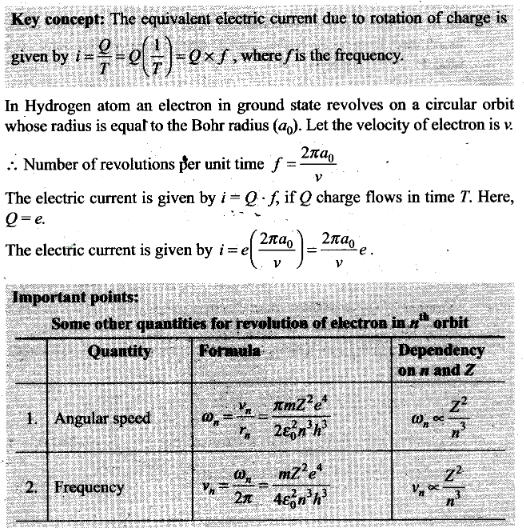
Question 22. Show that the first few frequencies of light that is
emitted when electrons fall to nth level from levels higher than n, are
approximate harmonics (i.e., in the ratio 1:2:3 …) when n >>
1.
Solution: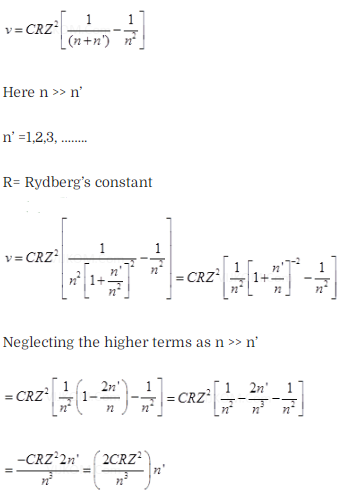
![]()
Question 23. What is the minimum energy that must be given to a
H-atom in ground state so that it can emit an Hґ Line in
Balmer series? If the angular momentum of the system is conserved, what would be
the angular momentum of
such Hґ photon?
Solution: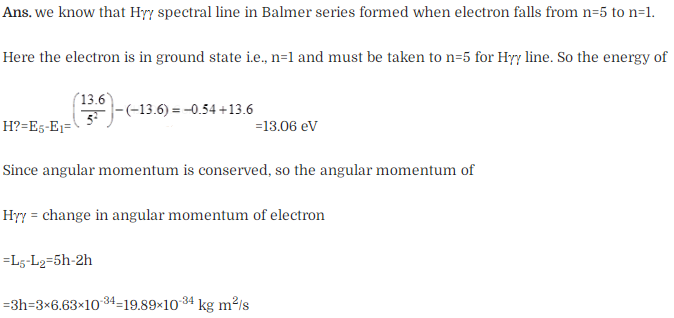
Long Answer Type Questions
Question 24. The first four spectral in the Lyman series of a H-atom
are λ= 1218 Å, 1028 Å, 974.3 Å and 951.4 Å. If instead of
Hydrogen, we consider deuterium, calculate the shift in the wavelength of these
lines.
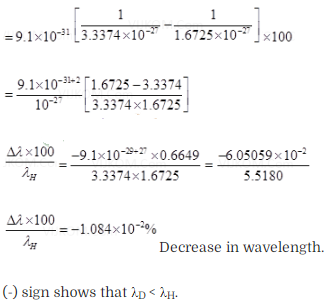
Solution: Let µH and
µD are the reduced masses of electron for hydrogen and deuterium
respectively.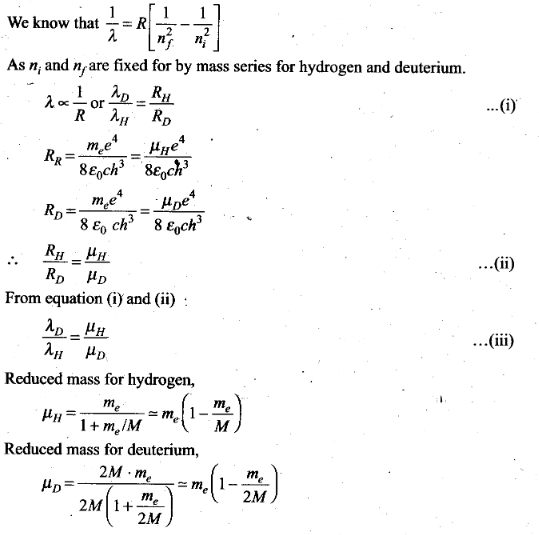
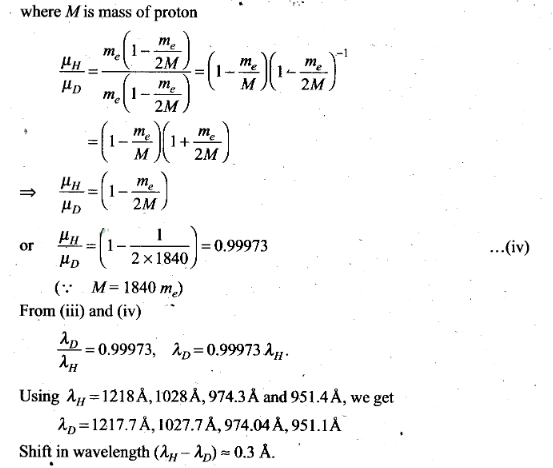
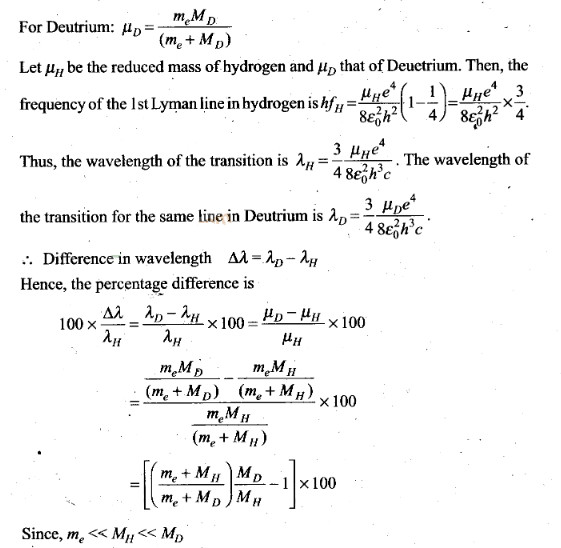
Question 25. Deutrium was discovered in 1932 by Harold Urey by
measuring the small change in wavelength for a particular transition
in 1H and 2H. This is because, the wavelength of
transition depend to a certain extent on the nuclear mass. If nuclear motion is
taken into account, then the electrons and nucleus revolve around their common
centre bf mass.
Such a system is equivalent to a single
particle with a reduced mass µ, revolving around the nucleus at a distance equal
to the electron-nucleus separation. Here µ=meMI(me +
M), wfiere M is the nuclear mass and me is the electronic mass.
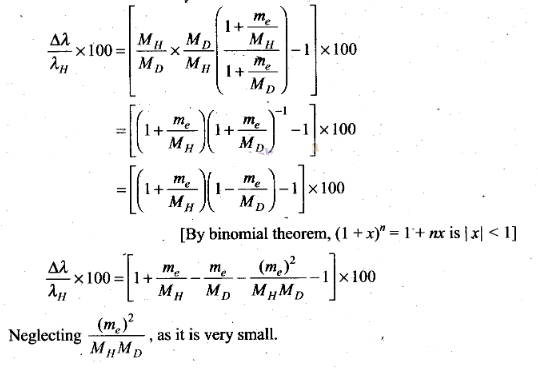
Estimate the percentage difference in wavelength for the 1st line of the Lyman
series in ‘H and 2H
(Mass of 1H
nucleus is 1.6725 x 10-27 kg, mass of 2H nucleus
is 3.3374 x 10-27 kg, Mass of electron = 9.109 x
10-31 kg.)
Solution: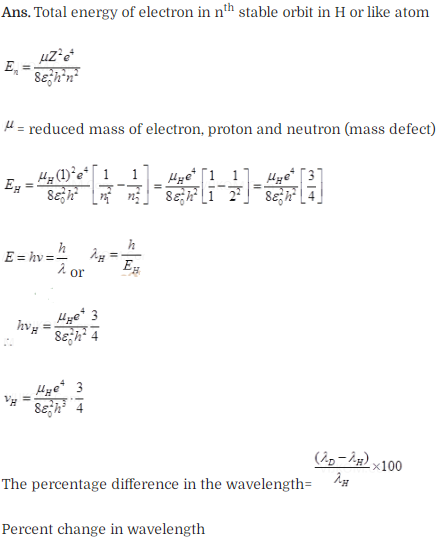

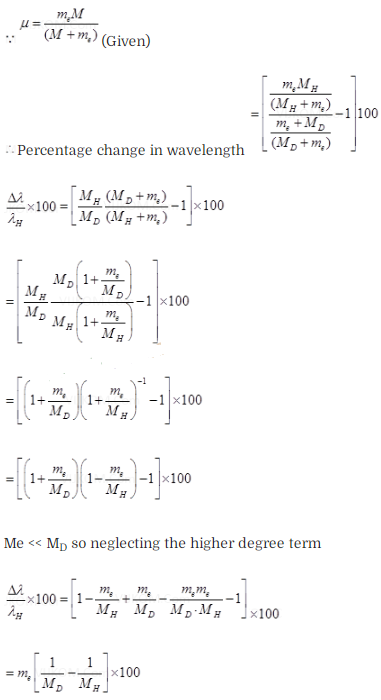
Question 26. If a proton had a radius R and the charge was uniformly
distributed, calculate using Bohr theory, the ground state energy of a H-atom
when (i) R = 0.1 Å and (ii) R = 10 Å .
Solution: In a H-atom in ground state, electron revolves round the
point-size proton in a circular orbit of radius rB (Bohr’s
radius).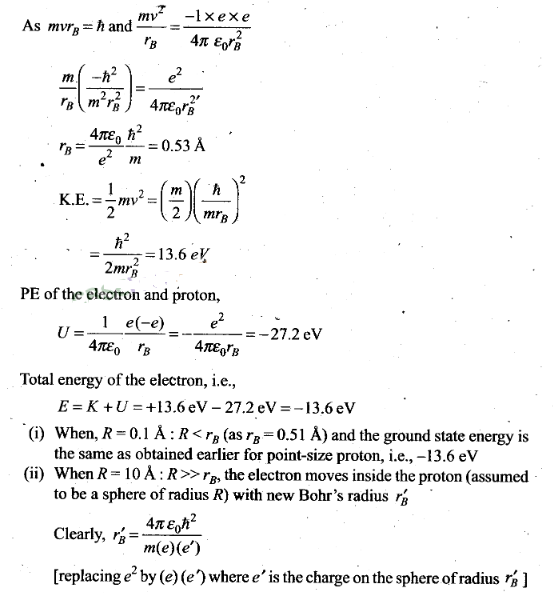
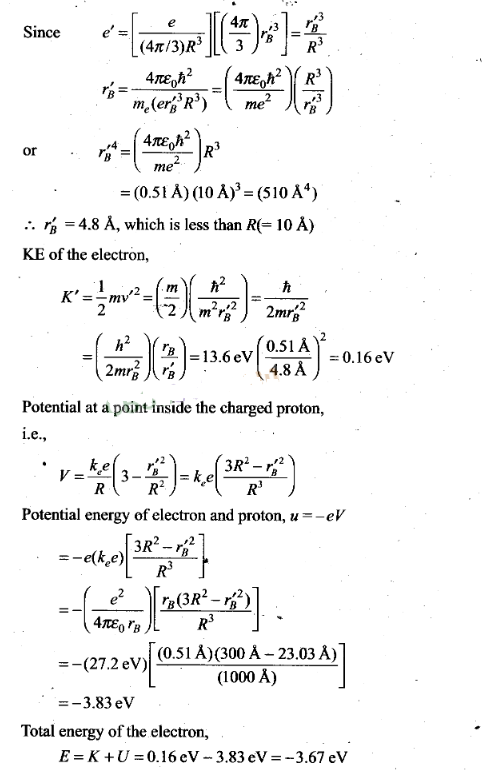
Question 27. In the Auger process, an atom maizes a transition to a
lower state without emitting a photon. The excess energy is transferred to an
outer electron which may be ejected by the atom (This is called an Auger
electron). Assuming the nucleus to be massive, calculate the kinetic energy of
an n = 4 Auger electron emitted by Chromium by absorbing the energy from a n – 2
to n = 1 transition.
Solution:
Key concept:
Auger Effect: The Auger effect is a process by which electrons with
characteristic energies are ejected from atoms in response to a downward
transition by another electron in the atom. In Auger spectroscopy, the vacancy
is produced by bombardment with high energy electrons, but the Auger effect can
occur if the vacancy is produced by other interactions. It is observed as one of
the methods of electron rearrangement after electron capture into the
nucleus.
If an inner shell electron is removed from an atom, an electron from
a higher level will quickly make the transition downward to fill the vacancy.
Sometimes this transition will be accompanied by an emitted photon whose quantum
energy matches the energy gap between the upper and lower level. Since for heavy
atoms this quantum energy will be in the x-ray region, it is commonly called
x.-ray fluorescence. This emission process for lighter atoms and outer electrons
gives rise to line spectra.
In other cases, the energy released by the
downward transition is given to one of the outer electrons instead of to a
photon, and this electron is then ejected from the atom with an energy equal to.
the energy lost by the electron which made the downward transition minus the
binding energy of the electron that is ejected from the atom. Though more
involved in interpretation than optical spectra, the analysis of the energy
spectrum of these emitted electrons does give information about die atomic
energy levels. The Auger effect bears some resemblance to internal conversion of
the nucleus, which also ejects an electron
Sometimes an upper election drops
to fill the vacancy, emitting a photon.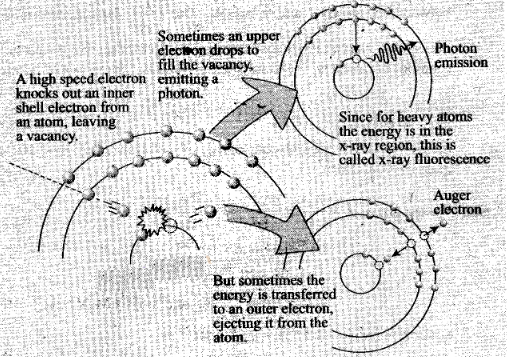
As the nucleus is massive, recoil momentum of the atom may be
neglected and the entire energy of the transition may be considered transferred
to the Auger electron. As there is a single valence electron in Cr, the energy
states may be thought of as given by the Bohr model.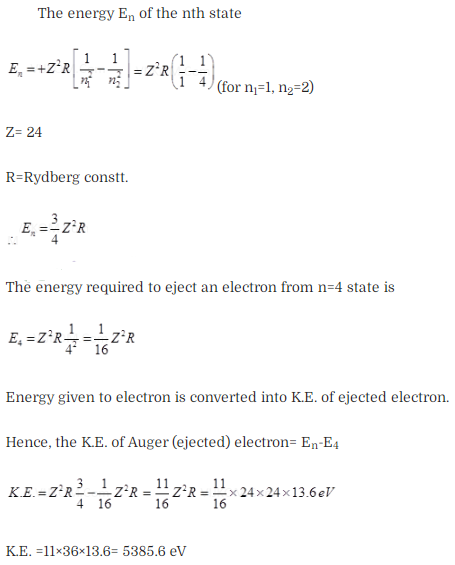
Question 28.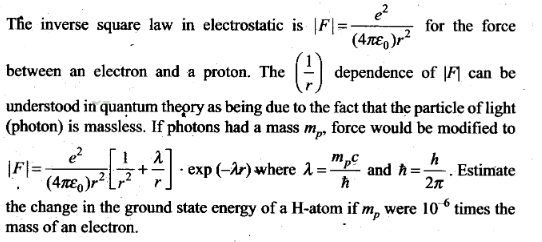
Solution: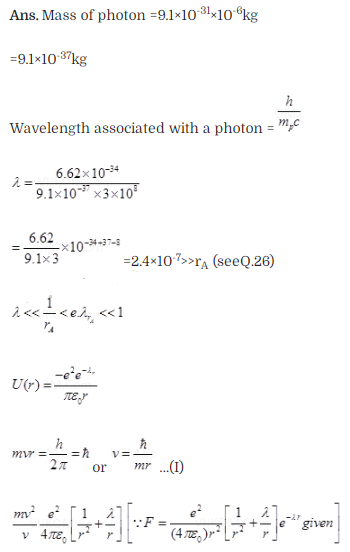


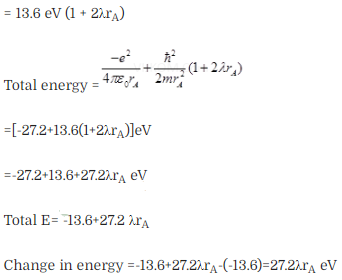
Question 29. The Bohr model for the H-atom relies on the Coulomb ’s
law of electrostatics. Coulomb’s law has not directly been verified for
very
short distances of the order of angstroms. Supposing
Coulomb’s law between two opposite charge +q1, – q2
modified to
Solution: