Dual Nature of Radiation and Matter
Class 12th Physics NCERT Exemplar Solution
Multiple Choice Questions (MCQs)
Single Correct Answer Type
Question 1. A
particle is dropped from a height H. The de-Broglie wavelength of the particle
as a function of height is proportional to (a) H (b)
H1/2 (c) H0 (d)
H-1/2
Solution: (d)
Key concept:
According to de-Broglie a moving material particle sometimes acts as a wave and
sometimes as a particle.
The wave associated with a moving particle is called
matter wave or de-Broglie wave and it propagates in the form of wave packets
with group velocity. According to de-Broglie theory, the wavelength of
de-Broglie wave is given by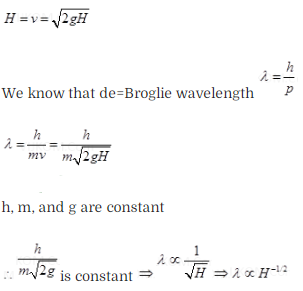
Question 2. The wavelength of a photon needed to remove a proton from
a nucleus which is bound to the nucleus with 1 MeV energy is
nearly
a) 1.2 nm (b) 1.2 x
10-3 nm
(c) 1.2 x
10-6 nm (d). 1.2 x 10 nm
Solution: (b)
Key concept: According to Einstein’s quantum theory
light propagates in the bundles (packets or quanta) of energy, each bundle being
called a photon and possessing energy.
Energy of photon is given by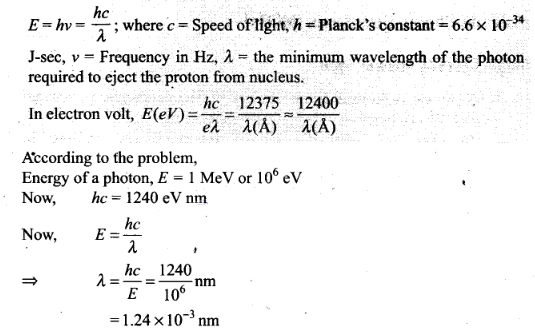
Question 3. Consider a beam of electrons (each electron with energy
E0) incident on a metal surface kept in an evacuated chamber.
Then,
(a) no electrons will be emitted as only photons can
emit electrons
(b) electrons can be emitted but all with an
energy, E0
(c) electrons can be emitted with any
energy, with a maximum of E0 – ɸ (ɸ is the work
function)
(d) electron can be omitted with energy ,with a
maximum of E0
Solution: (d) If a
beam of electrons of having energy E0 is incident on a metal surface
kept in an evacuated chamber.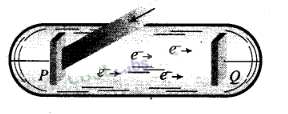
The electrons can be emitted with maximum energy
E0 (due to elastic collision) and With any energy less than
E0, when part of incident energy of electron is used in liberating
the electrons from the surface of metal.
Question 4. Consider the figure given below. Suppose the voltage
applied to A is increased. The diffracted beam will have the maximum at a
value of θ that (a) will be larger than the earlier value
(b) will be the same as the earlier value
(c) will be less
than the earlier value
(d) will depend on the
target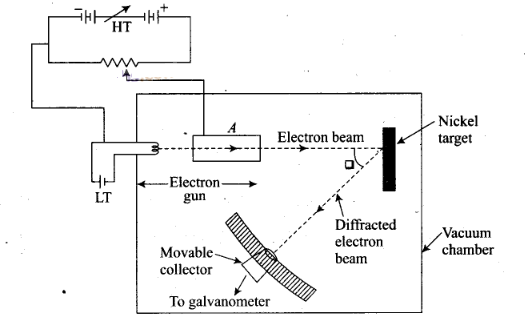
Solution: (c)
Key concept: Davision and
Germer Experiment:
1. It is used to study the scattering of electron from a
solid or to verify the wave nature of electron. A beam of electrons emitted by
an . electron gun is made to fall on nickel crystal cut along cubical axis at a
particular angle. Ni crystal behaves like a three dimensional diffraction
grating and it diffracts the electron beam obtained from electron gun.
2. The
diffracted beam of electrons is received by the detector which can be positioned
at any angle by rotating it .about the point of incidence. The energy of the
incideni beam of electrons can also be varied by changing the applied voltage to
the electron gun.
According to classical physics, the intensity of scattered
beam of electrons at all scattering angle will be same but Davisson and Germer
found that the intensity of scattered beam of electrons was not the same but
different at different angles of scattering. It is maximum for diffracting angle
50° at 54 volt potential difference.
3. If the de-Broglie waves exist for
electrons then these should be diffracted as X-rays. Using the Bragg’s formula
2d sinθ = nλ, we can determine the wavelength of these
waves.
The de-Broglie wavelength associated with electron is where V is the
applied voltage.
Using the Bragg’s formula we can determine the wavelength of
these waves. If there is a maxima of the, diffracted electrons at an angle θ,
then
2d sin θ = A (ii)
From Eq. (i), we note that if V is inversely
proportional to the wavelength λ. i.e., V will increase with
the decrease, in λ.
From Eq. (ii), we note that wavelength
λ is directly proportional to sinθ and hence θ.
So,
with the decrease in λ , θ will also decrease.
Thus, when the voltage applied to A is increased. The diffracted beam will have
the maximum at a value of θ that will be less than the earlier value.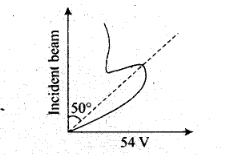
4. A proton, a neutron, an electron and an a-particle have
same energy. Then, their de-Broglie wavelengths compare as
Question 5. A proton, a neutron, an electron and an a-particle have
same energy. Then, their de-Broglie wavelengths compare as (a) λp =
λn > λe > λα
(b) λα < λp =
λn > λe
(c)
λe< λp = λn> λα (d)
λe = λp = λn = λα
Solution:
(b)
Key concept:
• Matter Waves (de-Broglie Waves)
According to
de-Broglie a moving material particle sometimes acts as a wave and sometimes as
a particle.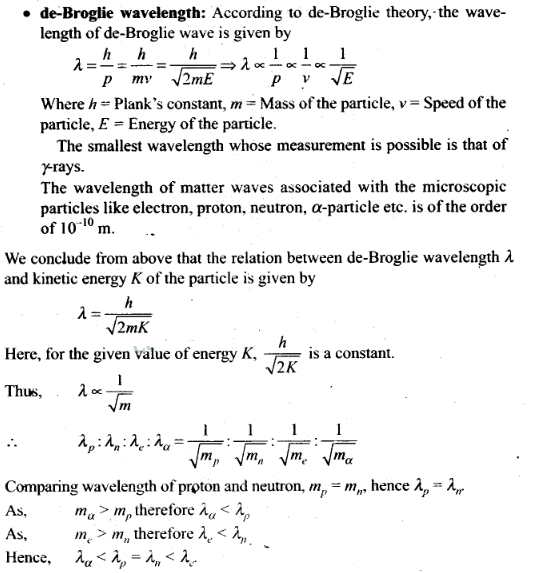
Question 6. An electron is moving with an initial velocity v =
v0i and is in a magnetic field B = B0j. Then, its
de-Broglie wavelength
(a) remains
constant
(b) increases with time
(c)
decreases with time
(d) increases and decreases
periodically
Solution: (a)
Key concept: If a
particle is carrying a positive charge q and moving with a velocity v enters a
magnetic field .5 then it experiences a force F which is given by the
expression
F = q(v x B)=$ F = qvB sin θ. As this force is perpendicular to v
and B , so the magnitude of v will not change, i.e. momentum (p = mv) will
remain constant in magnitude. Hence,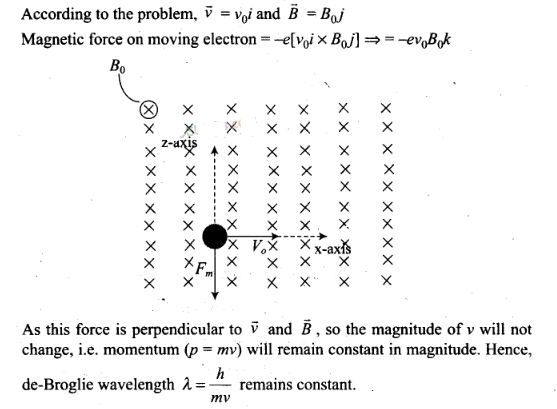
Question 7. An electron (mass m) with an initial velocity v =
v0i(v0 > 0) is in an electric field E =E0î
(E0 = constant > 0). Its de-Broglie wavelength at time t is given
by
Solution: (a)
Key concept: The wave
associated with moving particle is called matter wave or de-Broglie wave and it
propagates in the form of wave packets with group velocity. According to
de-Broglie theory, the wavelength of de- Broglie wave is given by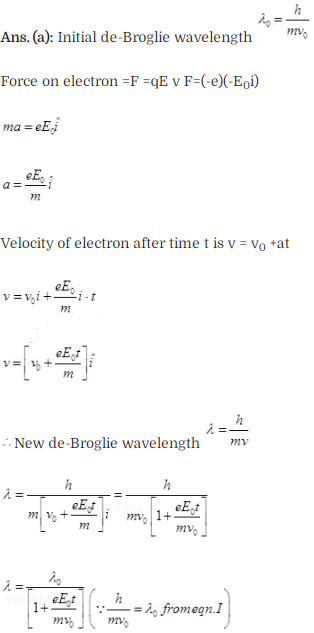
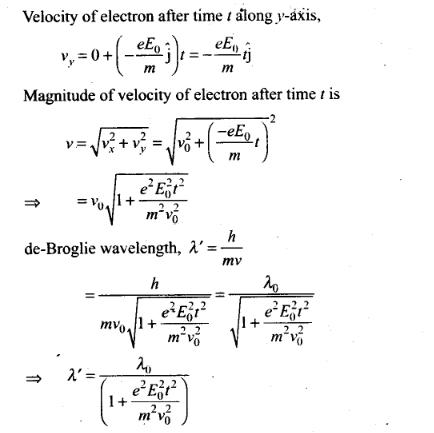
Question 8. An electron (mass m) with an initial velocity v
=v0î is in an electric field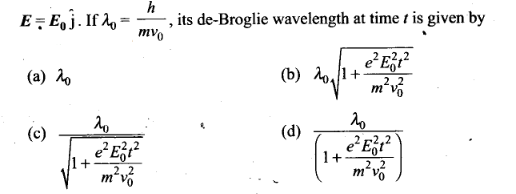
Solution: (c) According to the problem
de-Broglie wavelength of electron at time t=0.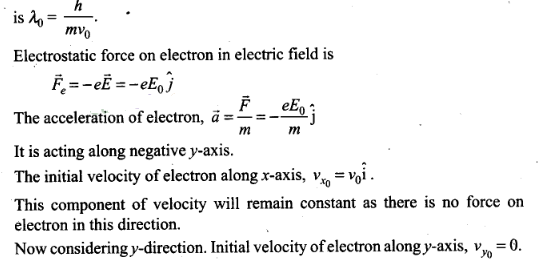
One or More Than One Correct Answer Type
Question 9. Relativistic corrections become necessary when the
expression for the kinetic
energy 1/2 mv2 ,
becomes comparable with mc2. where m is the mass of
the
particle. At what de-Broglie wavelength, will
relativistic corrections become important for an
electron?
(a) A=10nm (b) A =10-1 nm (c)
A=10-4 nm (d)
A=10-6 nm
Solution: (c, d)
Key
concept: De-Brogile or matter wave is independent of die charge on the material
particle. It means, matter wave of de-Broglie wave is associated with every
moving particle (whether charged or uncharged).
The de-Broglie wavelength at
which relativistic corrections become important that the phase velocity of the
matter waves can be greater than the speed of the light (3 x
108 m/s).
The wavelength of de-Broglie wave is given by
λ
= h/p = h/mv
Here, h = 6.6 x 10-34 Js
and for
electron, m = 9 x 10-31 kg
To approach these
types of problem we use hit and trial method by picking up each option one by
one.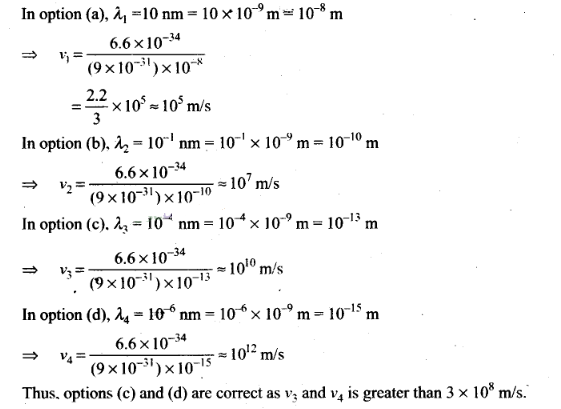
Question 10. Two particles A1 and
A2 of masses m1 , m2 (
m1> m2) have the same de-Broglie
wavelength. Then,
(a) their momenta are the same (b) their
energies are the same
(c) energy of A1 is
less than the energy of A2
(d) energy of
A1 is more than the energy
of A2
Solution: (a. c)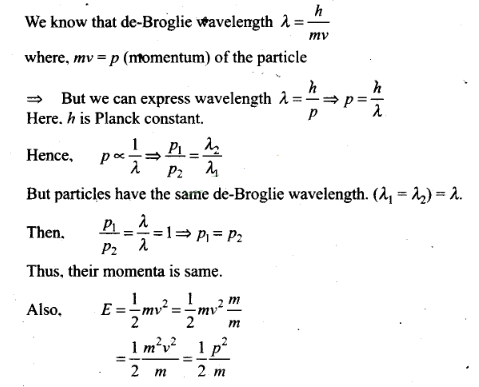

Question 11. De-Broglie wavelength associated with uncharged
particles: For Neutron efe-Broglie wavelength is given
as ve=c/100.Then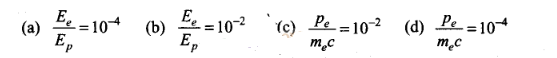
Solution:(b,c)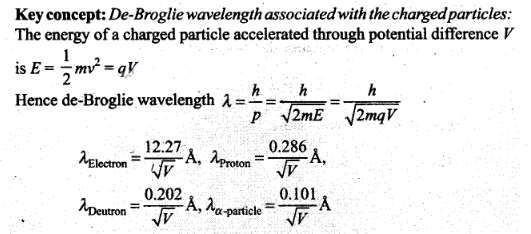

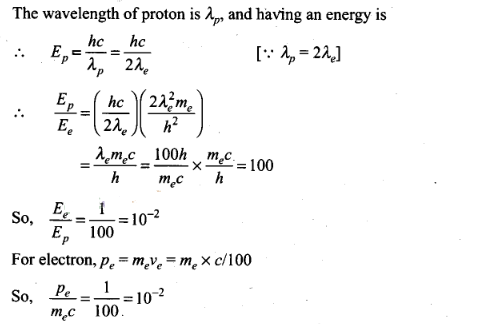

Question 12. Photons absorbed in a matter are converted to heat. A
source emitting v photon/sec of frequency v is used to convert 1 kg of ice at
0°C to water at 0°C. Then, the time T taken for the
conversion
(a) decreases with increasing n. with v
fixed
(b) decreases with n fixed, v
increasing
(c) remains constant with n and v changing such
that nv = constant
(d) increases when the product nv
increases
Solution: (a, b. c).
Question 13. A particle moves in a closed orbit around the origin,
due to a force which is directed towards the origin. The de-BrOglie wavelength
of the particle varies cyclically between two values λ1,
λ2 with λ1> λ2 Which
of the following statements are true?
(a) The particle
could be moving in a circular orbit with origin as centre
(b) The particle could be moving in an elliptic orbit with origin as its
focus
(c) When the de-Broglie wavetength is
λ1 the particle is nearer the origin than when its value
is λ2
(d) When the de-Broglie wavelength is
λ2 the particle is nearer the origin than when its value
is λ1
Solution: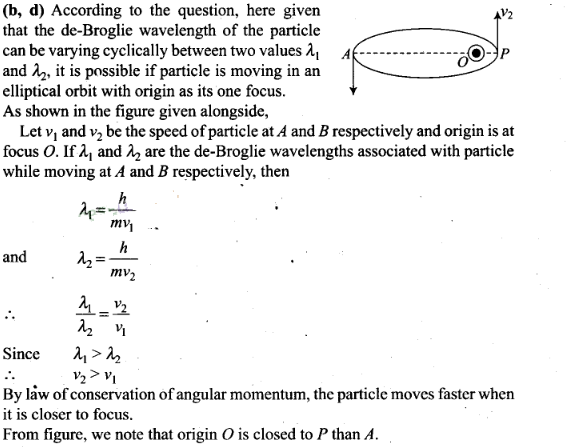
Very Short Answer Type Questions
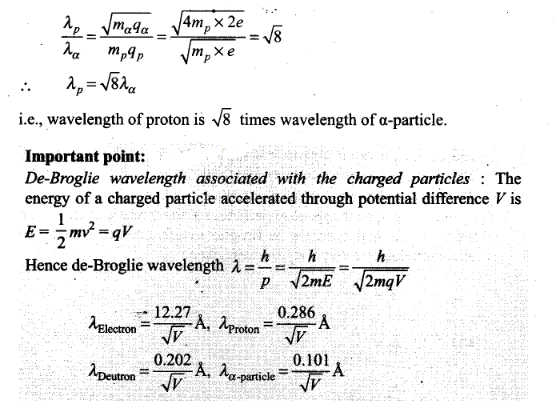
Question 14. Aproton and an a-particle are accelerated, using the
same potential difference. How are the de-Broglie wavelengths
λp and λα related to each
other?
Solution: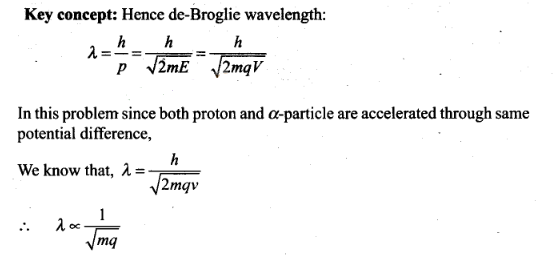
Question 15. (i) In the explanation of photoeletric effect, we assume
one photon of frequency v collides with an electron and transfers its energy.
This leads . to the equation for the maximum energy Emax of the
emitted electron as Emax = hv
– ɸ0
where ɸ0 is the work
function of the metal. If an electron absorbs 2 photons (each of frequency v),
what will be the maximum energy for the emitted electron?
(ii) Why is this fact (two photon absorption) not taken into consideration in
our discussion of the stopping potential?
Solution: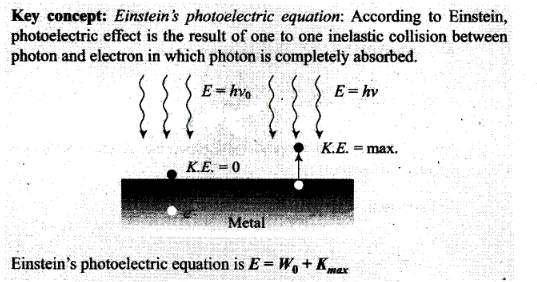
Question 16. There are materials which absorb photons of shorter
wavelength and emit photons of longer wavelength. Can there be stable substances
which absorb photons of larger wavelength and emit light of shorter
wavelength?
Solution: In the first case, when the
materials which absorb photons of shorter wavelength has the energy of the
incident photon on the material is high and the energy of emitted photon is low
when it has a longer wavelength or in short we can say that energy given out is
less than the energy supplied.
But in second case, the energy of the incident
photon is low for the substances which has to absorb photons of larger
wavelength and energy of emitted photon is high to emit light Of shorter
wavelength. This means in this statement material has to supply the energy for
the emission of photons.
But this is not possible for a stable
substances.
Question 17. Do all the electrons that absorb a photon come out as
photo electrons?
Solution:
Key concept:
Photo-Electric Effect:
The photo-electtic effect is the emission of electrons
(called photo-electrons when light strikes a surface. To escape from the
surface, the electron must absorb enough energy from the incident radiation to
overcome the attraction of positive ions in the material of the surface.
The
photoelectric effect is based on the principle of conservation of energy.
1.
Two conducting electrodes, the anode (Q) and cathode (P) are enclosed in an
evacuated glass tube as shown on next page.
2. The battery or other source of
potential difference creates an electric field in the direction from anode to
cathode.
3. Light of certain wavelength or frequency falling on the surface
of cathode causes a current in the external circuit called photoelectric
current.
4. As potential difference increases, photoelectric current also
increases till saturation is reached.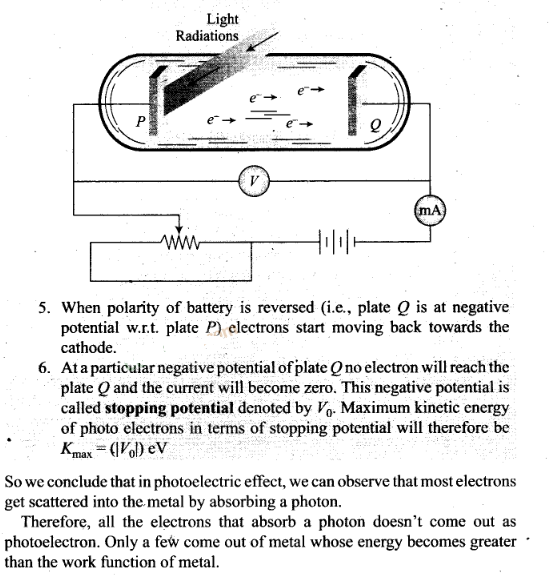
Question 18. There are two sources of light, each emitting with a
power of 100 W. One emits X-rays of wavelength 1 nm and the other visible light
at 500 nm. Find the ratio of number of photons of X-rays to the photons of
visible light of the given
wavelength.
Solution:
Key concept: X-Rays:
1.
X-rays were discovered by scientist Roentgen that is why they are also called
Roentgen rays.
2. Roentgen discovered that when pressure inside a discharge
tube is kept 10“3 mm of Hg and potential difference is kept 25 kV, then some
unknown radiations (X-rays) are emitted by anode.
3. There are three
essential requirements for the production of X-rays.
(i) A source of
electron
(ii) An arrangement to accelerate the electrons
(iii) A target of
suitable material of high atomic weight and high melting point on which these
high speed electrons strike.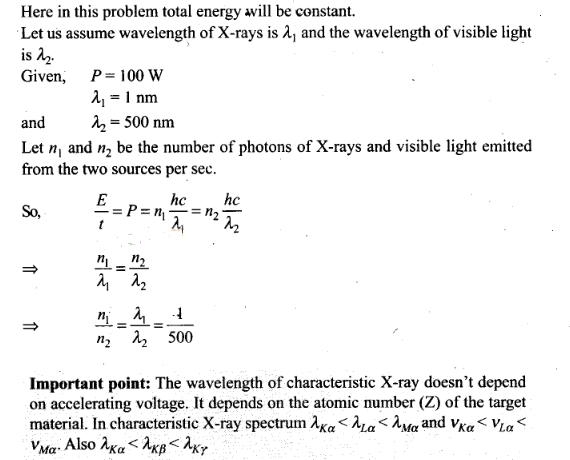
Question 19. Consider a metal exposed to light of wavelength 600 nm.
The maximum energy of the electron doubles when light of wavelength 400 nm is
used. Find the work function in eV.
Solution:The momentum of incident photon is
transferred to the metal ,during photo electric emission.
At microscopic
level ,atoms of a metal absorb the photon and its momentum is transferred mainly
to the nucleus and electrons.The excited electron is emitted.Therefore,the
conservation of momentum is to be considered as the momentum of incident photon
transferred to the nucleus and electron.
Question 20.Consider a metal exposed to light the wavelength of 600
nm. The maximum energy of electron doubles when light of wavelength 400 nm is
used.Find the work function in eV?
Solution: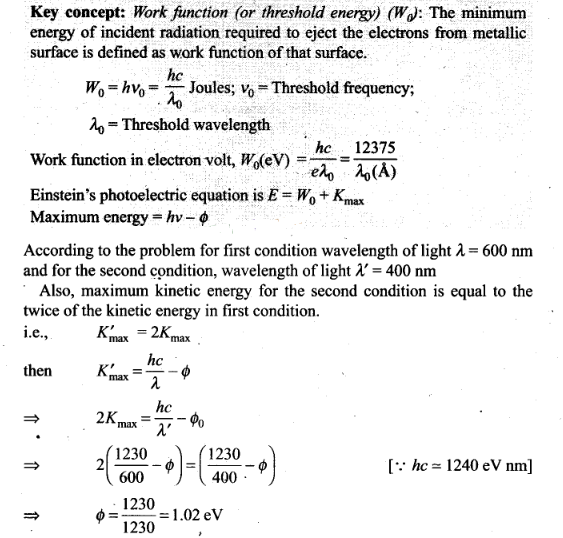
Question 21. Assuming an electron is confined to a 1 nm wide region,
find the uncertainty in momentum using Heisenberg uncertainty principle (∆x
x∆p=h). You can assume the uncertainty in position ∆x as 1 nm. Assuming p = ∆p,
find the energy of the electron in electron volts.
Solution: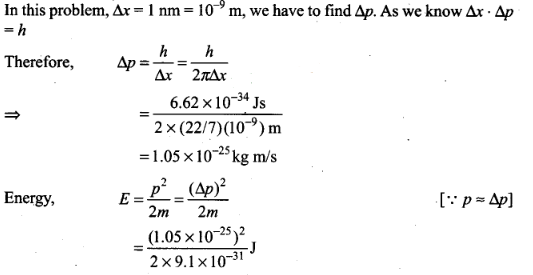
Question 22. Two monochromatic beams A and B of equal intensity I,
hit a screen. The number of photons hitting the screen by beam A is twice that
by beam B. Then, what inference can you make about their
frequencies?
Solution: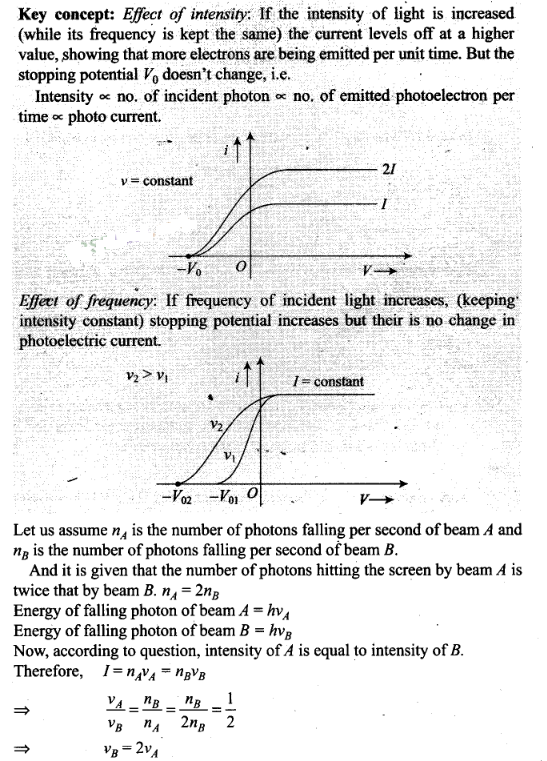
Question 23. Two particles A and B of de-Broglie wavelengths A, and
combine to form a particle C. The process conserves momentum. Find the
de-Broglie wavelength of the particle C. (The motion is
one-dimensional)
Solution: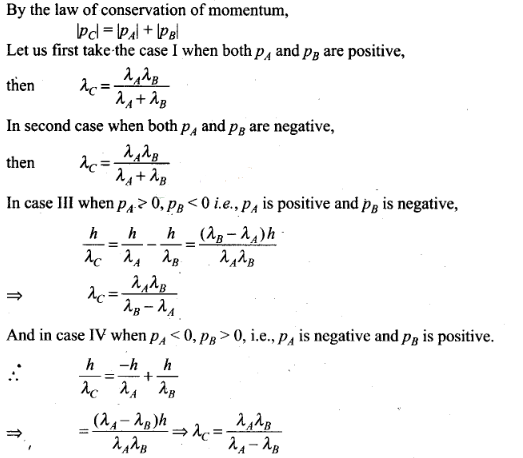
Question 24. A neutron beam of energy E scatters from atoms on a
surface with a spacing d = 0.1 nm. The first maximum intensity in the reflected
beam occurs at θ= 30°. What is the kinetic energy E of the beam in
eV?
Solution:
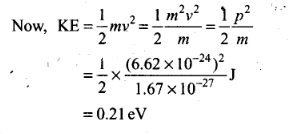
Long Answer Type Questions
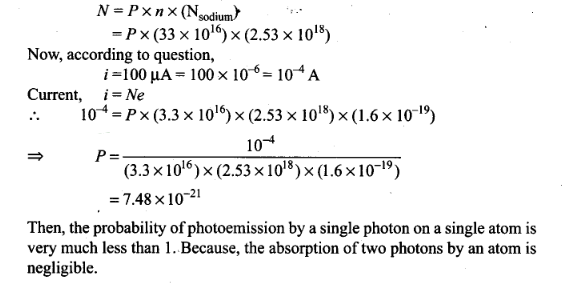
Question 25. Consider a thin target
(10-2 cm square,
10-3 m thickness) of sodium, which produces a photocurrent of 100 µA
when a light of intensity 100 W/m2 (λ = 660 nm) falls on it. Find the
probability that a photoelectron is produced when a photon strikes a sodium
atom. [Take density of Na = 0.97 kg/m3]
Solution:
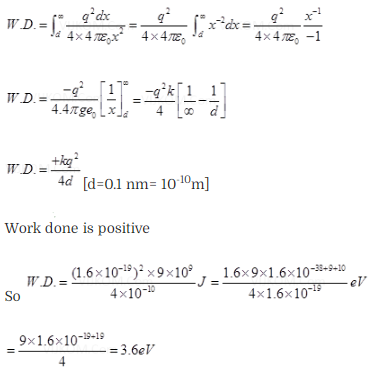
Question 26. Consider an electron in front of metallic surface of a
distance d.Assume the force of attraction by the plate is given as . Calculate
work in taking the to an infinite distance from the plate .Taking d=0.1 nm. find
the work done in electron volts?
Solution: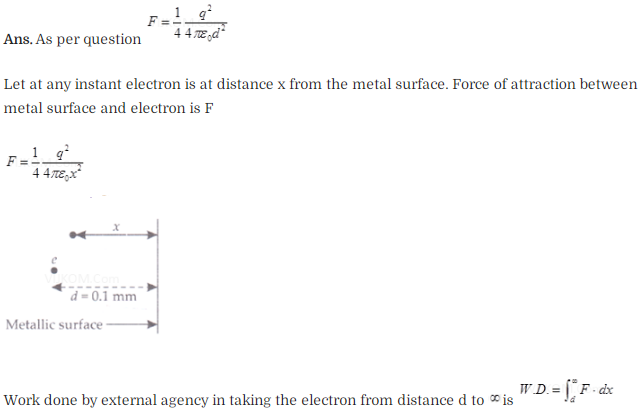
Question 27. A student performs an experiment on photoelectric
effect, using two materials A and B. A plot of Vstop versus v is
given in figure.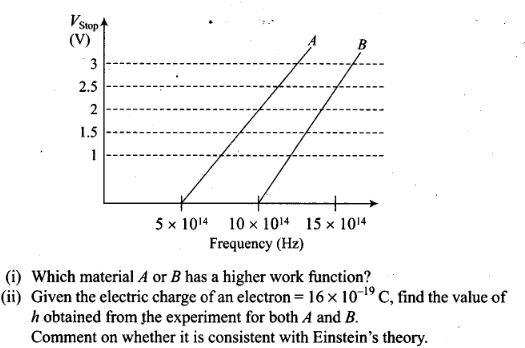
Solution: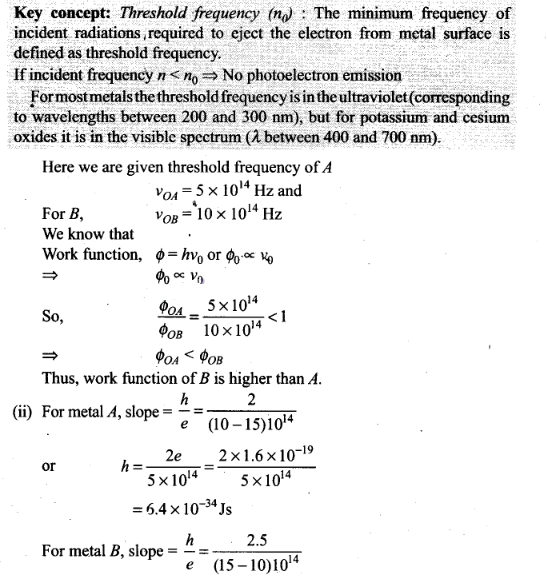
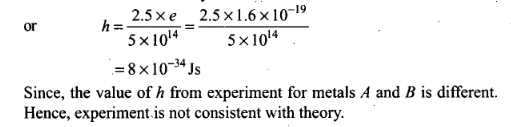
Question 28. A particle A with a mass mA is
moving with a velocity v and hits a particle B (mass mB) at rest (one
dimensional motion). Find the change in the de-Broglie wavelength of the
particle A. Treat the collision as elastic.
Solution:
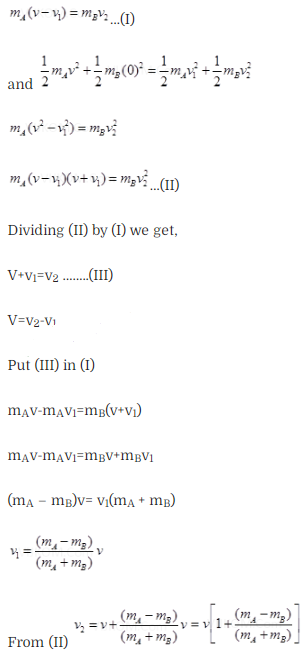
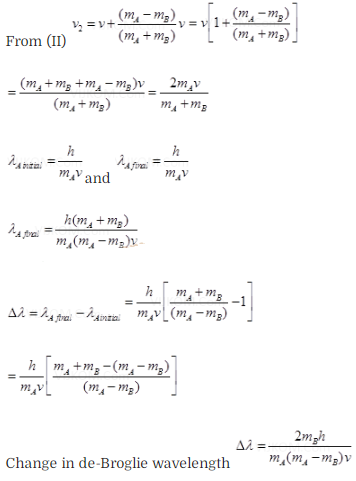
Question 29. Consider a 20 W bulb emitting light of wavelength 5000 Å
and shining on a metal surface kept at a distance 2 m. Assume that the metal
surface has work function of 2 eV and that each atom on the metal surface can be
treated as a circular disk of radius
1.5 Å.
(i) Estimate number of photons
emitted by the bulb per second. [Assume no other losses]
(ii) Will there be photoelectric emission?
(iii) How much
time would be required by the atomic disk to receive energy equal to work
function (2 eV)?
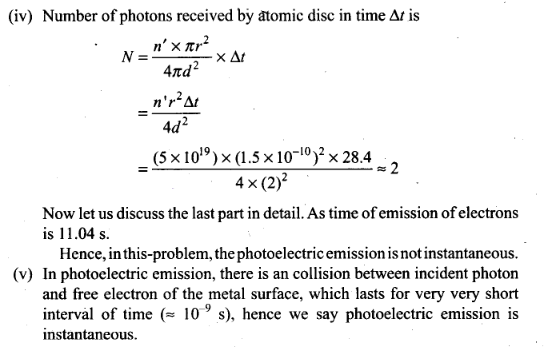
(iv) How many photons would atomic disk
receive within time duration calculated in (iii) above?
(v)
Can you explain how photoelectric effect was observed
instantaneously?
Solution: