CBSE Sample Papers for Class 12 Chemistry Set-8
Class 12thCBSE Sample Papers for Class 12 Chemistry Set-8
CBSE Sample Papers for Class 12 Chemistry Set 8 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section
A
(The following questions are
multiple-choice questions with one correct answer. Each question carries 1 mark.
There is no internal choice in this section.)
Question 1.
Which of the following reagent is required for the following
conversion ? [1]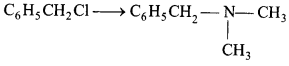
(a) CH3NH2, CH3Cl
(b)
CH3Cl, RNH2
(C) NH3, CH3Cl
(d)
CH3CH2NH2
Answer:
(a)
CH3NH2, CH3Cl
CH3NH2 and CH3Cl are the required reagents
for the given conversion.
Question 2.
Which of the following statements is not correct for
carboxylic acids? [1]
(a) Aromatic carboxylic acids undergo electrophilic
substitution reactions.
(b) Carboxylic acids are stronger acids than
alcohols.
(c) Carboxylic acids does not undergo any reaction with
Na2CO3 gas.
(d) Hell-Volhard Zelinsky reaction is given
by carboxylic acids.
Answer:
(c) Carboxylic acids does not undergo any
reaction with Na2CO3 gas.
When carboxylic acid reacts with Na2CO3 solution,
carbon dioxide is evolved with a brisk effervescence along with sodium
acetate.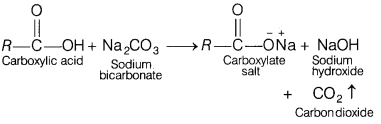
Question 3.
The major product of oxidation of secondary alcohol is [1]
(a) aldehyde
(b) ketone
(c) carboxylic acid
(d) ether
Answer:
(b)
ketone
Ketones can be prepared by the oxidation of secondary alcohols by using
oxidising agent such as K2Cr2O7/
H2SO4.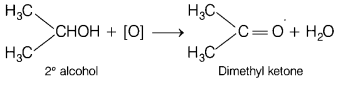
Question 4.
Arrange the following in order of increasing complexity of
chemical structures. [1]
A = Fructose, B = Starch, C = Maltose
(a) A <
B < C
(b) B < A <C
(c) B < C < A
(d) C < B < A
Answer:
(c) B < C < A
Fructose has molecular formula of C6H12O6.
Maltose has molecular formula of C12H22O11.
Starch has molecular formula of
(C6H10O5)n.
Hence, the increasing
order of complexity of chemical structures is![]()
Question 5.
Which of the following alkyl halides has maximum density?
[1]
(a) C3H7I
(b) C2H5I
(c)
CH3Br
(d) CH3I
Answer:
(d) CH3I
CH3I has maximum density because of smallest hydrocarbon part (i.e. CH3) and contain heaviest halogen (i.e. I).
![]()
Question 6.
In \(\left[\mathrm{CoF}_6\right]^{3-}\), Co3+ uses
outer d-orbitals (4d) in sp3d2-hybridisation. The number
of unpaired electrons present in complex ion is [1]
(a) 0
(b) 4
(c)
2
(d) 3
Answer:
(b) 4
[CoF6]3-
Here, Co is present in + 3 oxidation
state.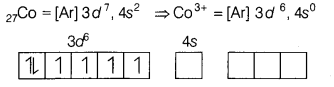
F being a weak ligand is unable to pair up its unpaired electrons thus, occupy
4s, 4p, and 4d empty orbitals as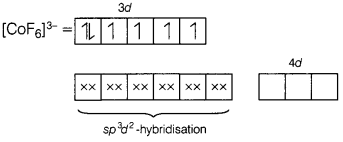
Thus, there are 4 unpaired electrons.
Question 7.
Match the properties with the metals. [1]
| (i) An element which has highest atomisation enthalpy. | (p) Mn |
| (ii) 3d-block element that show only +3 oxidation state. | (q) Zn |
| (iii) 3d-block element with lowest melting point. | (r) Sc |
| (s) Fe |
(a) (i) → (p), (ii) → (s), (iii) → (r)
(b) (i) → (s), (ii) → (q), (iii) →
(r)
(c) (i) → (s), (ii) → (r), (iii) → (q)
(d) (i) → (r), (ii) → (s),
(iii) → (p)
Answer:
(c) (i) → (s), (ii) → (r), (iii) → (q)
The correct match is (i)-(s), (ii)-(r), (iii)-(q)
(i) The enthalpy of
atomisation is highest for iron (Fe) in 3d/-series.
(ii) Scandium (Sc) have
only+3 oxidation state.
(iii) Zinc (Zn) has the lowest melting and boiling
point because it does not contain any d-electrons.
Question 8.
The following diagram shows the vapour pressure curves for
CH3F, CH3OH, CH3COOH and HCHO. [1]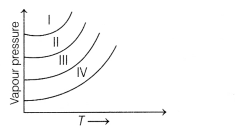
Curves I, II, III and IV respectively are for
(a)
CH3F; HCHO; CH3OH; CH3COOH
(b)
CH3COOH; CH3OH; CH3F; HCHO
(c) HCHO;
CH3FCH3OH; CH3COOH
(d) CH3OH;
CH3COOH; HCHO; CH3F
Answer:
(a) CH3F;
HCHO; CH3OH; CH3COOH
The vapour pressure increases with decrease in intermolecular interactions.
Moreover, lesser the intermolecular forces, more is the volatility and, hence
higher vapour pressure at a given temperature.
Therefore, CH3F has
highest vapour pressure, while CH3COOH has lowest vapour
pressure.
Thus, option (a) is correct.
Question 9.
In which case, Raoult’s law is not applicable? [1]
(a) 1 M
NaCl
(b) 1 M Urea
(c) 1 M Glucose
(d) 1 M Sucrose
Answer:
(a) 1 M
NaCl
Raoult’s law is not applicable, if the total number of particles of solute changes in the solution due to association or dissociation. Among the given compounds, NaCl undergoes dissociation and forms Na+ and Cl– ions. Therefore, Raoult’s law is not applicable to NaCl.
Question 10.
Which of the following will show a negative deviation from
Raoult’s law? [1]
(a) Acetone-benzene
(b) Acetone-ethanol
(c)
Benzene-methanol
(d) Acetone-chloroform
Answer:
(d)
Acetone-chloroform
Acetone and chloroform will show a negative deviation due to formation of
strong H-bonding after mixing and results in decrease vapour pressure.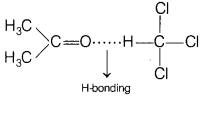
Question 11.
The diagram below shows an incomplete experimental set-up
needed to measure the Ecell of a cell composed of the standard
Cu2+ / Cu electrode and an Ag+ / Ag electrode. [1]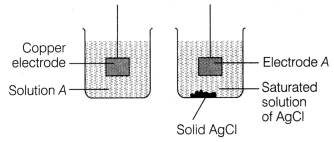
What is the chemical composition of solution A?
(a) CuSO4
(b)
AgSO4
(c) CuCl2
(d) AgCl
Answer:
(a)
CuSO4
Solution A = CuSO4
![]()
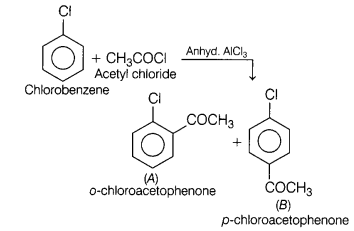
Question 12.
In the reaction, [1]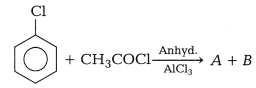
A and B are
(a) o-chloroacetophenone, methyl chloride
(b) p-chloroacetophenone, acetophenone
(c) o-chloroacetophenone,
p-chloroacetophenone
(d) None of the above
Answer:
(c)
o-chloroacetophenone, p-chloroacetophenone
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is
followed by a corresponding Reason (R). Use the following keys to choice the
appropriate answer.
(a) Both (A) and (R) are true and (R) is the correct
explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct
explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false,
but (R) is true.
Question 13.
Assertion (A) Crystal structure of oxides of transition
metals often show defects.
Reason (R) Ligand field effect causes distortions
in crystal structures. [1]
Answer:
(a) Both (A) and (R) are true and (R)
is the correct explanation of (A).
Question 14.
Assertion (A) o-nitrophenol is less soluble in water than the
m-and p-isomers.
Reason (R) m-and p-nitrophenols exist as associated
molecules. [1]
Answer:
(b) Both (A) and (R) are true, but (R) is not the
correct explanation of (A).
Due to the presence of intramolecular hydrogen bonding, o-nitrophenol does not form hydrogen bonds with H2O but m and p-nitrophenol form hydrogen bonds with water. Hence, o-nitrophenol is less soluble in water than the m and p-isomers.
Question 15.
Assertion (A) In order to convert R—Cl to pure
R—NH2, Gabriel phthalimide synthesis can be used.
Reason (R) With
proper choice of alkyl halides, phthalimide synthesis can be used to prepare 1°,
2° or 3° amines. [1]
Answer:
(c) (A) is true, but (R) is false.
(A) is true but (R) is false. It is because only primary aliphatic amines can be prepared by Gabriel-phthalimide reaction.
Question 16.
Assertion (A) Glucose does not form the hydrogen bisulphite
addition product.
Reason (R) Glucose is not so reactive to form the product
with NaHSO3. [1]
Answer:
(c) (A) is true, but (R) is false.
Glucose does not form the hydrogen bisulphite addition product because it has
cyclic structure in which —CHO group is not free to react.
Therefore, (A) is
true but (R) is false.
Section
B
(This section contains 5
questions with internal choice in one question. The following questions are very
short answer type and carry 2 marks each.)
Question 17.
A 4% solution (w/w) of sucrose (M = 342 g mol-1)
in water has a freezing point of 271.15 K. Calculate the freezing point of 5%
glucose (M = 180 g mol-1) in water. [2]
(Given : Freezing point of
pure water = 273.15 K)
Answer:
4% solution (w/w) of sucrose is
given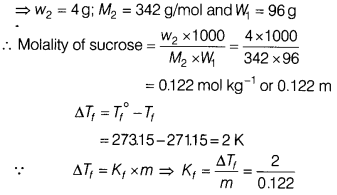
Now, molality of glucose solution
Given w2 = 5g; M2 =
180 g /mol; W1 = 95g
∴ Molality of glucose = \(\frac{W_2 \times
1000}{M_2 \times W_1}\)
= \(\frac{5}{180}\) × \(\frac{1000}{95}\)
= 0.292
m
∴ ∆Tf = Kf × m
= \(\frac{2}{0.122}\) × 0.292 =
4.8
∴ Freezing point of glucose solution = 273.15 – 4.8
= 268.35 K
Question 18.
Explain how and why will the osmotic pressure be affected
when external pressure applied becomes
(a) more than osmotic pressure.
(b)
less than osmotic pressure. [2]
Answer:
(a) When applied or external
pressure is more than osmotic pressure, then pure water is squeezed out by a
semipermeable membrane from the solvent, then the phenomenon of reverse osmosis
takes place.
(b) When external pressure is less than osmotic pressure, then the solvent starts flowing in to the solution through semipermeable membrane.
Question 19.
Account for the following. [2]
(a) Presence of a-hydrogen
in aldehydes and ketones is essential for aldol condensation.
Answer:
The
presence of alpha hydrogen in aldehydes and ketones is essential for aldol
condensation because they are acidic in nature (due to the presence of electron
withdrawing carbonyl group).
As a result, the electron density at α C— H bond decreases and hence, H-atom becomes weakly held which can be easily abstracted by strong bases forming enolate ion, which are stabilised by resonance.
(b) 3-hydroxypentan-2-one shows positive Tollen’s test.
Answer:
The
structure of 3-hydroxypentan-2-one is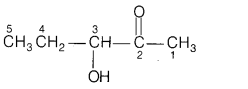
All α-hydroxy ketones gives Tollen’s test. Since, they have the ability to
tautomerises to aldehydes and aldehydes gives Tollen’s test.
As the given
compound is also α-hydroxy ketone and hence, it gives this test.
Question 20.
Give reason for the following. [2]
(a) Denaturation of proteins results in the loss of biological activity of
the proteins.
Answer:
During the denaturation of proteins, the secondary
and tertiary structures get destroyed and only the primary structure is
preserved. Covalent bonds are broken and interaction between amino acid chains
get disordered.
Consequently, denaturation leads to loss of biological
activity of proteins.
(b) Vitamins B and C cannot be stored in our body.
Answer:
Vitamins B
and C are soluble in water which must be supplied regularly in diet because they
are readily excreted in urine and cannot be stored (except vitamin
B12) in our body.
Or
(a) Name the deficiency diseases resulting from lack of vitamin A and E in
the diet. [2]
Answer:
The deficiency disease due to lack of vitamin A is
xerophthalmia and due to lack of vitamin E is increased fragility of RBCs and
muscular weakness.
(b) Out of the four bases, name those which are common to both DNA and RNA
and why DNA and RNA are called acids?
Answer:
Adenine, guanine and
cytosine are present in both DNA and RNA.
DNA and RNA are considered as acids
because they are formed from phosphate groups and these phosphates can readily
remove a proton and make DNA and RNA highly acidic.
Question 21.
When a coordination compound CoCl3 ∙
6NH3 is mixed with AgNO3, 3 moles of AgCl are precipitated
per mole of the compound. Write the
(a) structural formula of the
complex.
(b) IUPAC name of the complex. [2]
Answer:
(a) When one mole
of COCl3 ∙ 6H2O is mixed with AgNO3, three
moles of AgCl are precipitated which indicates that three ionisable chloride
ions in the complex are present.
Hence, its structural formula is
[Co(NH3)6]Cl3.
(b) IUPAC name of the complex [Co(NH3)6]Cl3 is hexaamminecobalt (III) chloride.
Section
C
(This section contains 7
questions with internal choice in one question. The following questions are
short answer type and carry 3 marks each.)
Question 22.
An organic compound with molecular formula
C9H10O, forms 2,4-DNP derivative, reduces Tollen’s reagent
and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzene
dicarboxylic acid. Identify the compound. [3]
Answer:
- As the given compound with molecular formula C9H10O, forms a 2,4-DNP derivative and reduces Tollen’s reagent, thus it must be an aldehyde.
- As it undergoes Cannizzaro reaction, hence —CHO group is directly attached to the benzene ring.
- On vigorous oxidation, it gives 1,2-benzene dicarboxylic acid.
Therefore, it must be an ortho-substituted , benzaldehyde and the only o-substituted aromatic aldehyde which have C9H10O molecular formula is o-ethyl benzaldehyde.
Question 23.
Write the equations for the following reaction. [3]
(a)
Benzyl chloride is treated with aqueous KOH followed by hydrolysis.
Answer: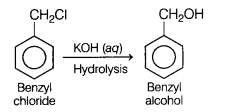
(b) Cumene hydroperoxide undergoes acid hydrolysis.
Answer: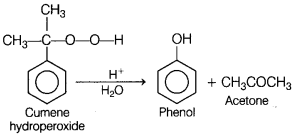
(c) Benzene diazonium chloride is treated with water.
Answer: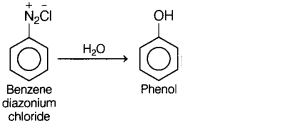
Question 24.
A steady current of 2 amperes was passed through two
electrolytic cells X and Y connected in series containing electrolytes
FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode
of cell X. How long did the current flow? Calculate the mass of Zn deposited at
the cathode of cell Y.
(Molar mass : Fe = 56 g mol-1
Zn = 65.3
g mol-1, 1F = 96500 C mol-1) [3]
Answer:![]()
∵ 56 g of Fe is deposited by 2F or 2 × 96500 C charge
∴
2.8 g of Fe will be deposited by
\(\frac{96500 \times 2 \times 2.8}{56}\) C
charge = 9650 C
Now, Q = lt
∴ Time, t = \(\frac{9650}{2}\) = 4825s
zn2+ + 2e– → zn
∵ Time, t = \(\frac{9650}{2}\) = 4825
s
zn2+ + 2e– → zn
∴ 2 × 96500 C charge deposits Zn =
65.3g
∴ 9650 C charge will deposit
Zn = \(\frac{65.3 \times 9650}{96500
\times 2}\) = 3.27 g
Question 25.
For the complex [Fe(en)2Cl2] Cl,
explain the
(a) type of hybridisation,
(b) magnetic behaviour and
(c)
number of its geometrical isomers. [3]
Answer:
(a)
[Fe(en)2Cl2]Cl
For the given compound, Fe is in + 3
oxidation state. Hence, its electronic configuration will be Fe3+ =
3d5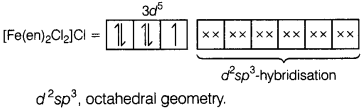
(b) Paramagnetic (as it contains one unpaired electron).
(c) Two geometrical isomers, cis and trans.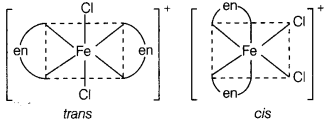
Question 26.
(a) Identify the products when D-glucose is treated with
Br2 water. [3]
(b) Why does the amino acids behave like salts
rather than simple amines or carboxylic acids?
Answer:
(a)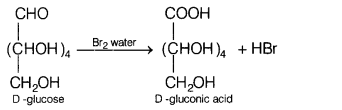
(b) In aqueous solution, the carboxyl group loses a proton and the amino
group accepts a proton. In other words, the carboxyl and amino groups neutralise
each other to form Zwitter ion. Due to formation of Zwitter ion, amino acids
behave as salts rather than simple amines or carboxylic acids.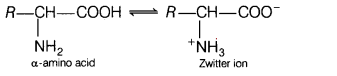
Or
(a) Name the carbohydrates which will yield saccharic acid on their reaction
with HNO3. Write the reaction involved.
(b) Glucose and fructose
are reducing sugars. Why? [3]
Answer:
(a)
It is saccharic acid which is
formed as per the reaction given below.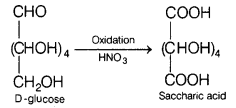
(b) Glucose and fructose are reducing sugars because they both contain a free aldehydic and ketonic group which makes them undergo oxidation readily to form carboxylic acid and in the process, the reactive reagents are reduced easily.
Question 27.
Answer the following questions. [3]
(a) A first order
reaction takes 40 min for 30% decomposition. Calculate the rate constant for
this reaction. (Given, log 1.428 = 0.1548)
Answer:
To find
t1/2, first calculate k by using the formula,
k =
\(\frac{2.303}{t}\)log\(\frac{[R]_0}{[R]}\)
For a reaction,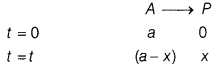
For 30% decomposition, it takes 40 min which means after 40
min. reactant reft is 70% of its initial concentration.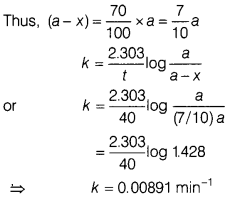
(b) Calculate t1/2 for this reaction.
Answer:
Half-life of
the reactant(t1/2)
= \(\frac{0.693}{k}\)
=
\(\frac{0.693}{0.00891}\)
= 77.75 min
(c) Explain why does the rate of a reaction not remain constant throughout
the reaction?
Answer:
∵ r ∝ [concentration]Order
Hence, as time increases rate decreases.
Question 28.
Give reason for the following observations.
(a) Chloroform
is stored in dark coloured bottles. [3]
Answer:
Chloroform is stored in
dark coloured bottles as in presence of light, it gets converted into highly
poisonous substance, phosgene (COCl2)
(b) Alkyl halides though polar are immiscible in water.
Answer:
To be
miscible with (or soluble in water) water, the solute-water force of attraction
must be stronger than the solute-solute and water-water forces of attraction.
Alkyl halides are polar molecules and so held together by dipole-dipole
interactions. Similarly, strong H-bonds exist between the water molecules.
The new force of attraction between the alkyl halides and water molecules is
weaker than the forces of attraction already existing between alkyl halide-alkyl
halide and water-water molecules.
Hence, alkyl halides (though polar) are
immiscible with (or insoluble in) water.
(c) In the pair of (CH3)3 C—Cl and CH3Cl,
CH3Cl will react faster in SN2 reaction with
\(\overline{\mathrm{O}}\) H.
Answer:
In SN2 reactions,
reactivity depends upon the steric hindrance CH3Cl being 1° halide
will react faster as compared to (CH3)3CCl which is a 3°
halide because 1° halides undergo SN2 mechanism faster than 3°
halides.
Section
D
(The following questions are
case-based questions. Each question has an internal choice and carries 4(1+1+2)
marks each. Read the passage carefully and answer the questions that
follow.)
Question 29.
Amines constitute one of the most important class of organic
compounds. Amines are alkyl or aryl derivatives of ammonia formed by replacement
of one or more hydrogen atoms. Alkyl derivatives are called aliphatic amines and
aryl derivatives are known as aromatic amines. Both aliphatic and aromatic
primary amines can be prepared by the reduction of nitro compounds catalytically
with H2 in the presence of active metal in acidic medium.
The presence of aromatic amines can be identified by performing dye test. Aniline is the simplest example of aromatic amine. It undergoes electrophilic substitution reactions in which —NH2 group strongly activates the aromatic ring through delocalisation of lone pair of electrons of N-atom.
Aniline undergoes electrophilic substitution reactions. Ortho and para-positions to the —NH2 group become centres of high electron density.
Thus, —NH2 group is ortho and para-directing powerful activating group.
Answer the following questions. [4]
(a) Propylamine reacts with which reagent to form an alkali soluble product?
(1)
Answer:
Propylamine reacts with Hinsberg reagent to form an alkali
soluble product, i.e. N-propylbenzene
sulphonamide.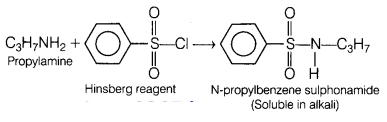
(b) Which compound is produced by oxidation of aniline with
K2Cr2O7/H2SO4 ?
Answer:
The compound formed as a product is p-benzoquinone, when oxidation of
aniline is carried out with K2Cr2O7 in presence
of H2SO4.
The reaction involved is as follows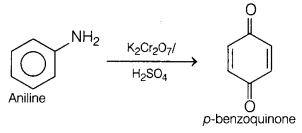
(c) Convert ethyl iodide to ethyl amine. Name all the compound involve in
various steps.
Answer:
The series of reaction that takes place during the
conversion of ethyliodide to ethylamine is as follows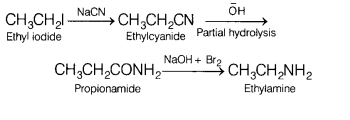
Or
Suggest a route by which the following conversion can be accomplished. Also
with the name of all the compound involve in the steps. [4]
Answer:
Complete conversion can be performed as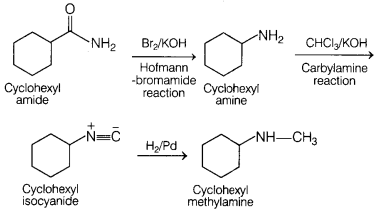
Question 30.
For any chemical reaction, thermodynamics tells about the
feasibility of a reaction chemical equilibrium tells about the extent to which a
reaction will proceed and chemical kinetics tells about the rate at which a
chemical reaction proceeds and factors which control this rate. When one or more
substances undergo a change which results in the formation of a new products, it
is called chemical reaction order and molecularity are the properties of a
reaction, which helps in understanding its mechanism.
Answer the following questions. [4]
(a) For the reaction A → B, then rate of reaction becomes three times when
the concentration of A is increased by nine time. What is the order of
reaction?
Answer:
r1 = k[A]x ……. (i)
r2 = k[9A]x ….. (ii)
On dividing Eq. (ii) by Eq. (i),
we get
\(\frac{r_2}{r_1}\) = \(\frac{k[9 A]^x}{k[A]^x}\) ⇒ 3 =
(9)x ⇒ (3)1 = (32)x
⇒ 2x = 1 ⇒ x = 1/2
Thus, the order of reaction is 1/2.
Or
For a reaction A + B → P, the rate law is given by, r = k[A]2
[B]2. What is the order of this reaction? [4]
Answer:
For a
reaction, A + B → P
Given, rate of a reaction, r = k
[A]1/2[B]2
Order w.r.t. A = \(\frac{1}{2}\);
Order
w,r.t. B = 2
∴ Overall order of a reaction = 1/2 + 2 = 5/2
(b) Identify the order of a reaction, if the units of its rate constant
are
(i) L-1 mol
(ii) L mol-1 s-1
Answer:
The units of rate constant depends upon the order of reaction.
(i)
Zero order L-1mol s-1
(ii) Second order L
mol-1 s-1
(c) Discuss any four factors which affect the rate of a chemical
reaction.
Answer:
Factors influencing the rate of a chemical reaction
are
- Nature of reactants: Different reactants require different amount of energies for breaking the old bonds and for the formation of new bonds. Hence, the reactivity of a substance is related to the ease with which the specific bonds are broken or formed.
- Concentration of reactants: Rate of reaction is directly proportional to the concentration of the reactants.
- Temperature: Rate of reaction increases with increase in temperature.
- Catalyst: It alters the rate of reaction without being consumed in the reaction. It provides an alternative path to the reaction with a low energy barrier.
Section
E
(The following questions are
long answer type and carry 5 marks each. All questions have an internal
choice.)
Question 31.
(a) Why does the conductivity of a solution decreases with
dilution? [5]
Answer:
Conductivity of a solution is related with the
number of ions present per unit volume of the solution. When the solution is
diluted, the number of ions per unit volume decreases. Hence, conductivity or
specific conductance of the solution decreases.
(b) Write the reaction involved in the working of H2 –
O2 fuel cell.
Answer:
The electrode reactions involved in the
working of H2 – O2 fuel cell are as follows At
cathode,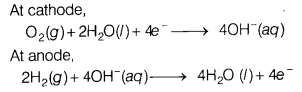
(c) What is the potential of hydrogen electrode which is in contact of a
solution whose pH is 10?
Answer: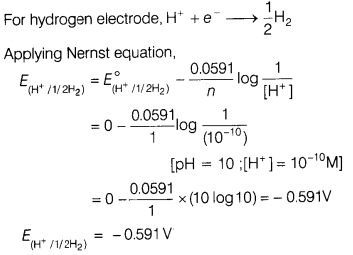
Or
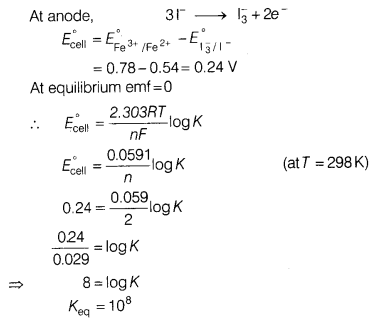
(a) Calculate the equilibrium constant for the reaction, [5]
2Fe3+ + 3I– \(\rightleftharpoons\) 2Fe2+ +
\(\mathrm{I}_3^{-}\)
The standard reduction potential in acidic condition is
0.78 V and 0.54 V respectively, for Fe3+/Fe2+ and
\(\mathrm{I}_3^{-}\) /I– couples.
Answer:
(b) For a cell,
Ag(s) | AgNO3 (0.01 M) || AgNO3 (1.0
M) | Ag(s)
What is the emf of the cell at 25°C?
Answer:
Electrochemical
reaction is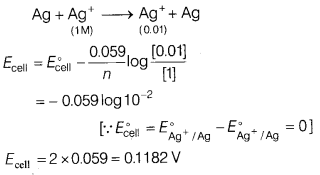
Question 32.
Attempt any five of the following. [5]
(a) Why
Cu+ ion is not known in aqueous solution?
Answer:
In aqueous
solution, copper (I) undergoes disproportionation reaction.
2Cu+
(ag) → Cu2+ + Cu(s)
The highest stability of Cu2+ ion
in aqueous solution is due to negative enthalpy of hydration.
Hence,
Cu+ is not known (or unstable) in aqueous solution.
(b) Why Mn3+ is a good oxidising agent?
Answer:
The outer
electronic configuration of Mn3+ is d4 configuration. It
has maximum tendency to gain one electron to change into stable half-filled
configuration, i.e. d5. Therefore, it is the strongest oxidising
agent.
(c) Why Ni2+ is more stable than Pt2+ whereas
Pt4+ is more stable than Ni4+ ?
Answer:
Ni2+ compounds have the sum of first two ionisation enthalpies to be
lower than that of Pt2+ compounds and are thermodynamically
stable.
However, the sum of first four ionisation enthalpies of
Pt4+ is lower than that of first four IEs of Ni4+ and is
relatively stable.
(d) Explain the observation, Zn, Cd and Hg are quite soft and have low
melting point.
Answer:
Zn, Cd, Hg metals have completely filled d-orbitals
(d10). It means thatd-electrons are not readily available for
metallic bond formation. Since, the metallic bonds are weak.
Therefore, these
metals are quite soft and also have low melting points.
(e) What is meant by ‘disproportionation reactions’?
Answer:
The
disproportionation reactions are those in which the same substance gets oxidised
as well as reduced.
When a particular oxidation state becomes less stable
relative to other oxidation states (one lower and one higher), it undergoes
disproportionation.
(f) Complete and balance the reaction.
Fe2+ +
Mn\(\mathrm{O}_4^{-}\) + H+ →
Answer:
5Fe2+ +
Mn\(\mathrm{O}_4^{-}\) + 8H+ → Mn2+ + 5Fe3+ +
4H2O
(g) Why transition metals exhibit variable oxidation states?
Answer:
ns
and (n – 1)d electrons of transition metal have very little difference in the
energies and hence both can participate in bonding, which results in variable
oxidation states.
When ns electrons take part in bonding, they exhibit lower oxidation states whereas when (n – 1)d electrons along with ns electrons participate in bonding, they exhibit variable oxidation states.
question 33.
An organic compound (A) having molecular formula
C2H6O on oxidation with
Na2Cr2O7 /H2SO4 produces
a compound (B) which reduces Tollen’s reagent.
Both (A) and (B) produce a
yellow solid on treatment with I2 / OH–. Identify A and B
and write all the involved reactions. [5]
Answer:
Since, an organic
compound (A) with molecular formula C2H6O on oxidation
with Na2Cr2O7/H2SO4 gives
compound (S) which reduces Tollen’s reagent, therefore, (A) must be ethanol and
(B) must be ethanal (S).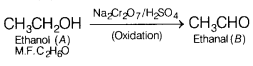
Since ethanol (A), CH3CH2OH contains
the group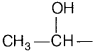
and ethanal (B), CH3CHO contain the group
CH3CO—, therefore, both these on treatment with I2 /
OH– undergo iodoform reaction to give yellow solid of
iodoform.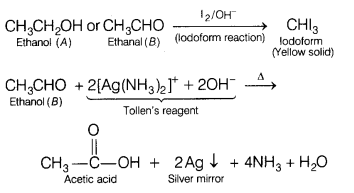
Or
A hydrocarbon, P, with the formula C6H12 readily decolourises bromine. [5]
(a) On reaction with hot, concentrated, acidified potassium manganate (VII)
solution a single organic product, Q, is obtained. Q gives an orange precipitate
when reacted with 2, 4-dinitrophenylhydrazine, 2, 4-DNP reagent, but has no
reaction with Tollen’s reagent.
(i) Identify P and Q and write their IUPAC
names.
(ii) Write down the reaction for the formation of Q from P.
(iii)
Write down the reaction to prepare propanol from compound Q.
Answer:
(i)
Decolourisation of bromine shows that P has a C = C double bond and is an
alkene.
A single organic product on reaction with hot, concentrated acidified
potassium manganate (VII) implies P is symmetrical.
Reaction with 2, 4-DNP
implies 0 contains a carbonyl group.
No reaction with Tollen’s reagent
implies that 0 is a ketone and not an aldehyde.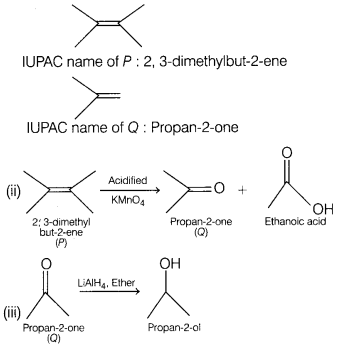
(b) Arrange the following compounds in the increasing order of their property
as indicated.
(i) Acetaldehyde, acetone, di-ferf-butyl ketone, methyl
fert-butyl ketone (reactivity towards HCN).
(ii) Benzoic acid, 4-nitrobenzoic
acid, 3, 4-dinitrobenzoic acid, 4 methoxy benzoic acid (acid strength).
Answer:
(i) Di-tertiary butyl ketone < methyl tertiary butyl ketone <
acetone < acetaldehyde.
(ii) 4-methoxy benzoic acid < benzoic acid <
4-nitrobenzoic acid < 3, 4-dinitrobenzoic acid.