CBSE Sample Papers for Class 12 Chemistry Set-6
Class 12thCBSE Sample Papers for Class 12 Chemistry Set-6
CBSE Sample Papers for Class 12 Chemistry Set 6 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section
A
(The following questions are
multiple-choice questions with one correct answer. Each question carries 1 mark.
There is no internal choice in this section.)
Question 1.
Zero order reaction can be represented graphically as
follows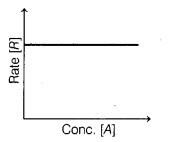
The (i) slope and (ii) rate if the graph are [1]
(a) (i)
ln[A] (ii) k
(b) (i) zero (ii) k
(c) (i) -[A] (ii) zero
(d) (i) zero
(ii) -[A]
Answer:
(b) (i) zero (ii) k
For zero-order reaction,
Rate = k[A]° = k
The rate does not change with
time.
Thus, the slope will be zero and rate = k
Question 2.
The number of ions produced from
[Co(NH3)6]Cl3 in solution is [1]
(a) 3
(b) 6
(c) 9
(d) 4
Answer:
(d) 4
Four ions are produced from [Co(NH3)6]Cl3 in
solution.
[CO(NH3)6]Cl3
\(\rightleftharpoons\) [Co(NH3)6]+ +
3Cl–
Question 3.
The conversion of benzaldehyde into methylbenzene by
zinc-amalgam and hydrochloric acid is called as, [1]
(a) Wolff-Kishner
reaction
(b) Rosenmund reaction
(c) Clemmensen reaction
(d) Birch
reaction
Answer:
(c) Clemmensen reaction
Clemmensen reaction involves the reduction of aldehyde or ketone to alkane
using Zn-Hg and hydrochloric acid.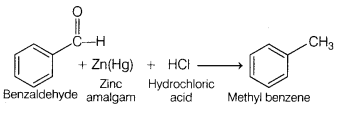
Question 4.
Which one of the following aldehydes does not give Cannizzaro
reaction? [1]
(a) Formaldehyde
(b) Acetaldehyde
(c) Thmethyl
acetaldehyde
(d) Benzaldehyde
Answer:
(b) Acetaldehyde
Cannizzaro reaction is given by the aldehydes that do not contain α-H atom. The aldehydes like acetaldehyde, CH3CHO possess α-H atom and hence, does not undergo Cannizzaro reaction.
Question 5.
What would be the major product of the following reaction?
[1]
C6H5SO2Cl +
C2H5NH2 → A + B
(a) A =
C6H5SO2NH2, B =
C2H5Cl
(b) A =
C6H5SO3H, B =
C2H4NH2Cl
(c) A =
C6H5SO2C2H5, B =
NH3
(d) A =
C6H5SO2NHC2H5, B =
HCl
Answer:
(d) A =
C6H5SO2NHC2H5, B =
HCl
The reaction of benzene sulphonyl chloride with primary amine yields N-ethyl
benzene sulphonamide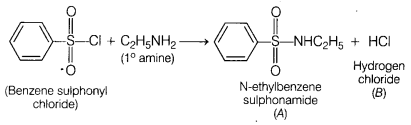
Question 6.
When sodium tert-butoxide and methyl iodide are heated, the
major product formed is [1]
(a) dimethyl ether
(b) fert-butyl methyl
ether
(c) di-fert-butyl ether
(d) isobutylene
Answer:
(b) tert-butyl
methyl ether
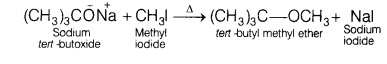
Question 7.
Which one of the following compounds will give butanone on
oxidation with alkaline KMnO4 solution? [1]
(a) Butan-l-ol
(b)
Butan-2-ol
(c) Butene
(d) But-2-ene
Answer:
(b) Butan-2-ol
Butan-2-ol on oxidation with alkaline KMnO4 solution produces
butanone. Complete reaction is as follows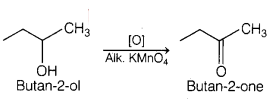
Question 8.
The compound which reacts fastest with Lucas reagent is (at
room temperature) [1]
(a) butan-1-ol
(b) butan-2-ol
(c)
2-methylpropan-1-ol
(d) 2-methylpropan-2-ol
Answer:
(d)
2-methylpropan-2-ol
When Lucas reagent is treated with 1°, 2° and 3° alcohols, then turbidity appears. If turbidity is appeared immediately, then alcohol is tertiary. 2-methyl propan-2-ol is a tertiary alcohol. Hence, it reacts fastest with Lucas reagent.
Question 9.
Match the items of Column I and Column II. [1]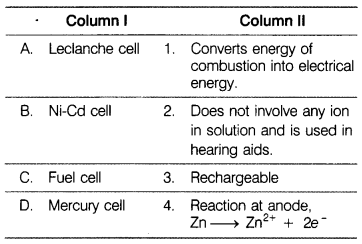
Answer:
(a)![]()
(a) A – (4),
B – (3),
C – (1),
D – (2).
Question 10.
The coordination number of central metal ion in
[Fe(C2O4)3]3- is [1]
(a) 4
(b)
5
(c) 6
(d) 3
Answer:
(c) 6
C2O4 is a bidendate ligand and its value is 3 in the complex. So, the coordination number will be 3 × 2 = 6.
Question 11.
What is the order of a reaction that is 50% complete after 2h
and 75% complete after 4h? [1]
(a) First order
(b) Second order
(c)
Zero order
(d) None of the above, information given insufficient
Answer:
(a) First order
It is a first order reaction because for 75% completion of reaction two half-lives are required (as, t1/2 = 2h) which suggests that t1/2 is independent of initial concentration.
Question 12.
Which of the following statement is incorrect? [1]
(a)
Ethers are less soluble than corresponding alcohols.
(b) Ethers are polar in
nature.
(c) O-atom in ether is sp-hybridised.
(d) Ether has net dipole
moment.
Answer:
(c) O-atom in ether is sp-hybridised.
The incorrect statement is (c) because O-atom in ethers show sp3-hybridisation due to the presence of two sigma bond pairs and two lone pair of electrons over it.
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is
followed by a corresponding Reason (R) use the following keys to choose the
appropriate answer.
(a) Both (A) and (R) are true, (R) is the correct
explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct
explanation of (A).
(c) (A) is true, (R) is false.
(d) (A) is false, (R)
is true.
Question 13.
Assertion (A) Zero order reactions are relatively uncommon
but these occur under special conditions.
Reason (R) Thermal decomposition of
HI on gold surface is a zero order reaction. [1]
Answer:
(b) Both (A) and
(R) are true, (R) is not the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
Zero order reactions are not so common and they occur under special conditions,
like some enzyme catalysed reactions and reactions which occur on metal surface
are few examples of zero order reactions.
Moreover, thermal decomposition of
HI on gold surface is also zero order reaction.
Question 14.
Assertion (A) Order of basicity of amines in gaseous phase is
NH3 > primary amine > secondary amine > tertiary amine.
Reason (R) In gaseous phase, the basicity of amine depends upon the stability of
ammonium cation formed by accepting proton from water. [1]
Answer:
(d) (A)
is false, (R) is true.
Order of basicity of amines in gaseous phase follows the order
Tertiary
amine > secondary amine > primary amine > NH3
The basic
nature of aliphatic amines increases with increase in the number of alkyl groups
(in gaseous phase). Hence, (A) is false, (R) is true.
Question 15.
Assertion (A) Tollen’s reagent, Benedict’s solution and
Fehling solution are reducing agents.
Reason (R) Mild oxidising agents like
chlorine or bromine water convert glucose into gluconic acid. [1]
Answer:
(d) (A) is false, (R) is true.
Benedict’s solution, Tollen’s reagent, Fehling’s solution etc., are mild oxidising agents. They oxidise the aldoses and ketoses to the corresponding acids and get themselves reduced. Thus, (A) is false but (R) is true.
Question 16.
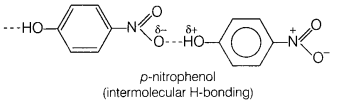
Assertion (A) o-nitrophenol is less volatile than
p-nitrophenol.
Reason (R) There is intramolecular hydrogen bonding in
o-nitrophenol and intermolecular hydrogen bonding in p-nitrophenol. [1]
Answer:
(d) (A) is false, (R) is true.
(A) is false but (R) is true, o-nitrophenol is more volatile due to
intramolecular hydrogen bonding, while p-nitrophenol is less volatile due to
intermolecular hydrogen bonding which causes the association of
molecules.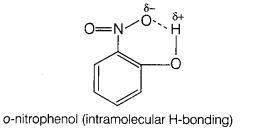
Section
B
(This section contains 5
questions with internal choice in one question. The following questions are very
short answer type and carry 2 marks each.)
Question 17.
How are the following conversions carried out?
(a) Benzoic
acid to m-nitrobenzyl alcohol. [2]
Answer:
Bezoic acid to m-nitrobenzyl
alcohol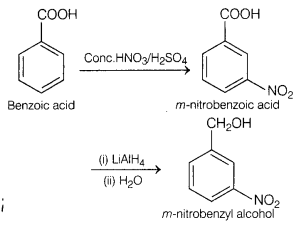
(b) Propene to propan-2-ol.
Answer:
Propene to propan-2-ol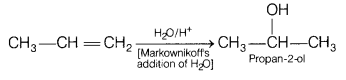
Question 18.
Give reasons for the following.
(a) Measurement of osmotic
pressure method is preferred for the determination of molar masses of
macromolecules such as proteins and polymers. [2]
Answer:
The osmotic
pressure method has the advantage over other methods as pressure measurement is
around the room temperature and the molarity of the solution is used instead of
molality.
(b) Elevation of boiling point of 1 M KCl solution is nearly double than that
of 1 M sugar solution.
Answer:
Elevation in boiling point is directly
proportional to ‘i’ ∆Tb ∝ i. Now, as given in the question, elevation
of boiling point of 1 M KCI solution is nearly double than that of 1 M sugar
solution. It is because KCI being ionic, dissociates into K+ and
Cl– and therefore it’s van’t Hoff factor, (i) is 2, whereas for sugar
van’t Hoff factor is 1 as it does not undergoes such a dissociation.
Or
(a) Name and state the law for a solution containing volatile components.
[2]
Answer:
Raoult’s law For a solution of volatile liquids, the partial
vapour pressure of each component of the solution is directly proportional to
its mole fraction present in solution. Thus, for any component, partial vapour
pressure,
p ∝ χ ⇒ p = p°χ
where, p° is the vapour pressure of pure
component and χ is the mole fraction of that component.
(b) Out of 1 M glucose and 2 M glucose, which one has a higher boiling point
and why?
Answer:
2 M glucose has higher boiling point because more the
concentration, more is the elevation in boiling point.
Question 19.
The formula [Pt(en)2Cl2] represent a coordination compound.
Write IUPAC name and structures of possible isomers. [2]
Answer:
[Pt(en)2Cl2]
IUPAC name
Dichloridob/’s(ethylenediamine)
platinum(II). Geometrical isomers of
[Pt(en)2Cl2] are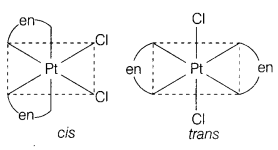
Question 20.
Explain how and why
(a) aquatic species are more
comfortable in cold water than warm water? [2]
Answer:
Oxygen is present
in dissolved state in water. As per Henry’s law when temperature rises
solubility of a gas decreases in solvent, it means solubility of oxygen in warm
water is less than cold water. This makes aquatic species respirate comfortably
in cold water.
(b) at higher altitudes, people suffer from anoxia resulting in inability to
think?
Answer:
At higher altitude people suffer from anoxia because at
higher altitudes, the partial pressure of oxygen is less than that at ground
level. This leads to low concentration of oxygen in the blood and tissues of
people.
Question 21.
(a) The molar conductivity of a 1.5 M solution of an
electrolyte is found to be 138.9 S cm2/ mol. What is the conductivity
of the solution? [2]
Answer:
Molar conductivity, \(\Lambda_m\) = 138.9 S
cm2 mol-1
Molarity = 1.5 M
\(\Lambda_m\) =
\(\frac{\kappa \times 1000}{C}\)
\(\kappa\) = \(\frac{\Lambda_{\mathrm{m}}
\times C}{1000}\) = \(\frac{138.9 \times 1.5}{1000}\)
\(\mathbf{K}\) = 0.208
cm-1
(b) Why does a salt-bridge is used in a galvanic cell? [1]
Answer:
Salt-bridge connects the solution of two half-cells to complete the cell circuit
and it prevents the transference or diffusion of the solutions from one half
cell to other.
Section
C
(This section contains 7
questions with internal choice in one question. The following questions
are
short answer type and carry 3
marks each.)
Question 22.
Write the equations for the following reactions. [3]
(a)
Methyl chloride is treated with AgNO2.
Answer: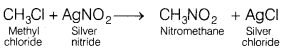
The reaction between methyl chloride and AgNO2
produces nitromethane and silver chloride. This reaction is an example of
substitution reaction.
(b) Bromobenzene is treated with CH3Cl in the presence of
anhydrous AlCl3.
Answer: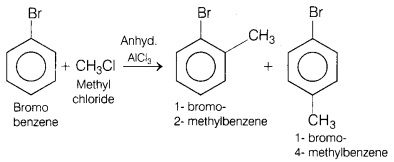
This reaction involves Friedel-Crafts alkylation.
When bromobenzene is
treated with CH3Cl in the presence of anhydrous AlCl3, it
gives 1 -bromo-2-methylbenzene and-1 -bromo-4-methyl benzene as major
product.
(c) Ethyl chloride is treated with aqueous KOH.
Answer:
Ethyl chloride
undergoes hydrolysis to form ethyl alcohol (through SN2 nucleophilic
substitution).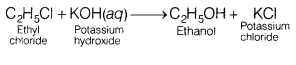
Question 23.
(a) Identify the product formed when propene undergoes
anti-Markowinikoff’s addition. Name the reagent which is used to carry out the
reaction. [3]
Answer: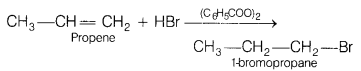
This reaction is an example of anti-Markownikoff’s addition in which negative part of hydrohalide goes to that carbon which is having more number of hydrogen in the presence of peroxide.
(b) Why do haloalkanes easily dissolve in organic solvents?
Answer:
Haloalkanes easily dissolve in organic solvents because both are covalent in
nature (like dissolve like). Also the steric effect in haloalkanes is not more
as seen in the case of haloarenes.
Or
(a) Name the product formed when p-nitrochlorobenzene is heated with aqueous
NaOH at 443 K, followed by acidification. Write the reactions involved.
Answer: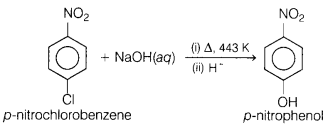
In this reaction, p-nitrochlorobenzene is converted into p-nitrophenol when
reacted with NaOH in the presence of heat followed by acidification.
(b) Butan-1-ol is optically inactive but butan-2-ol is optically active.
Why?
Answer:
Butan-1 -ol is optically inactive compound due to the absence
of asymmetric C-atom (or chiral C-atom) whereas butan-2-ol is optically active
compound due to the presence of asymmetric C-atom (or chiral C-atom).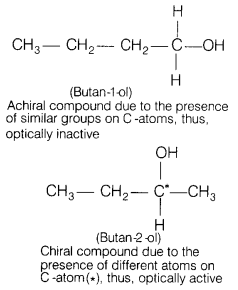
Question 24.
Give reasons of the following observations.
(a) Gabriel
phthalimide synthesis is not preferred for synthesising aromatic primary amines.
[3]
Answer:
Aryl halides do not undergo nucleophilic substitution with
salt formed by phthalimide. That’s why Gabriel phthalimide synthesis is not
preferred to prepare aromatic primary amines.
(b) Tert-butylamine cannot be prepared by the action of NH3 on
tert-butyl bromide.
Answer:
Ammonolysis (action of NH3) gives
the highest yield with 1° halides where substitution predominates because 10
halides behave as nucleophile While in case of 3° halide elimination
predominates where 3° halide acts as base.
Therefore, tert-butylamine cannot be prepared by the action of NH3
on tert-butyl bromide.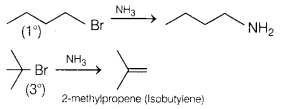
(c) Acetylation of aniline reduces its activation effect.
Answer:
Due
to electron withdrawing effect of the acetyl group, the lone pair of electrons
on N-atom is attracted by acetyl group.
As a result, lone pair of electrons
on N-atom is not exclusively available for donation to the benzene ring and
hence, activating effect of the —NH2 group is reduced.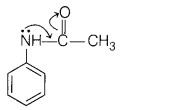
Question 25.
How are vitamins classified? Name the vitamin responsible for
the coagulation of blood. [3]
Answer:
Classification of vitamins Vitamins
are classified into two groups depending upon their solubility in water or
fat
- Fat soluble vitamins
- Water soluble vitamins
Vitamin K is responsible for the coagulation of blood.
Question 26.
Using crystal field theory, draw energy level diagram, write
electronic configuration of central atom/ion and determine the magnetic moment.
[3]
(a) [FeF6]3-
Answer:
The electronic
configuration of Fe3+ is [Ar]3d5. Since F– is a
weak field ligand, ∆0 < P. Therefore, a high spin complex is
formed. The orbital diagram of [FeF6]3- is as
follows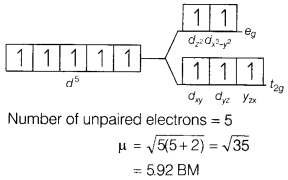
(b) [Fe(H2O)6]2+
Answer:
[Fe(H2O)6]2+
The electronic configuration of
Fe2+ is [Ar]3d6.
Since, H2O is also a weak
field ligand, ∆0 < P. Therefore, a high spin complex is formed.
The orbital diagram of Fe(H2O)2+ is as follows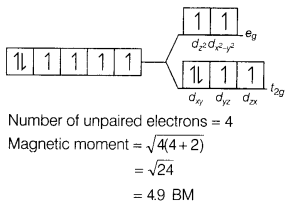
(c) [Fe(CN)6]4-
Answer:
[Fe(CN)6]4-
The electronic configuration of
Fe2+ is [Ar]3d6. But CN– is strong field
ligand. Thus, pairing of electron will takes place, ∆0 > P.
Therefore, a low spin complex is formed. The orbital diagram of
[Fe(CN)6]4- s as follows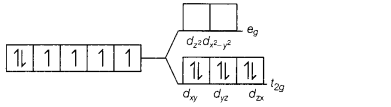
No unpaired electrons, so diamagnetic.
Magnetic moment = 0
Question 27.
Answer the following questions. [3]
(a) Henry’s law is for a solution containing volatile components. Then how
does Raoult’s law become a special case of Henry’s law?
Answer:
In the
solution of a gas in a liquid, one of the components is so volatile that it
exists as a gas and its solubility is given by Henry’s law which states
that,
P = KHχ.
i.e. partial pressure of the volatile component
(gas) is directly proportional to the mole fraction of that component (gas) in
the solution.
When the equations of Raoult’s law and Henry’s law are
compared, it can be seen that the partial pressure of the volatile component or
gas is directly proportional to its mole fraction in solution. Only the
proportionality constant KH differs from p°.
(b) Assume that N2 exerts a partial pressure of 0.987 bar. If
N2 gas is bubbled through water at 293 K, then what will be its mole
fraction? Given that Henry’s law constant for N2 at 293 K is 76.48
kbar.
Answer:
According to Henry’s law,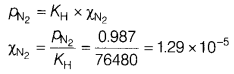
Question 28.
Amino acids may be acidic, alkaline or neutral. How does this
happen? What are essential and non-essential amino acids? Name one of each type.
[3]
Answer:
Amino acids may be acidic, basic or neutral depending upon the
relative number of amino and carboxyl group present in the molecule.
Equal
number of amino and carboxyl groups makes it neutral.
More number of amino
groups than carboxyl groups makes it basic and more carboxyl groups as compared
to amino groups makes it acidic.
- Essential amino acids are the a-amino acids which are needed for health and growth of human beings but are not synthesised by the human body, e.g. valine, leucine.
- Non-essential amino acids are the a-amino acids which are needed for health and growth of human beings and are synthesised by the human body, e.g. glycine, aspartic acid.
Section
D
(The following questions are
case-based questions. Each question has an internal choice and carries 4(1+1+2)
marks each. Read the passage carefully and answer the questions that
follow.)
Question 29.
Within the 3d-series, manganese exhibits oxidation states in
aqueous solution from +2 to +7. Likewise, iron forms both Fe2+(ag)
and Fe3+(ag). Cr and Mn form oxy-ions Cr\(\mathrm{O}_4^{2-}\),
Mn\(\mathrm{O}_4^{-}\), owing to their willingness to form multiple bonds.
The pattern with the early transition metals in the 3d series up to Mn, and
for the 4d, 5d metals up to Ru and Os is that the maximum oxidation state
corresponds to the number of “outer shell” electrons.
The highest oxidation
states of 3d metals may depend upon the complex formation (e.g. the
stabilisation of Co3+ by ammonia) or upon the pH (thus
Mn\(\mathrm{O}_4^{2-}\)(ag) is prone to disproportionation in acidic solution).
Within the 3d-series, there is considerable variation in relative stability of
oxidation states, sometimes on moving from one metal to a neighbour; thus, for
iron, Fe3+ is more stable than Fe2+, especially in
alkaline conditions, while the reverse is true for cobalt.
The ability of transition metals to exhibit a wide range of oxidation states is marked with metals such as vanadium, where the standard potentials can be rather small, making a switch between states relatively easy.
Answer the following questions. [4]
(a) In 3d-series, Mn exhibits highest oxidation state while in 4d and 5d, Ru
and Os has the highest oxidation state. What is the highest oxidation state
among the given elements?
Answer:
Ru and Os exhibit highest state in their
respective series. Both shows + 8 oxidation state (Mn show) +7.
Or
Although zirconium belongs to 4 d-transition series and hafnium to
5d-transition series even then they show similar physical and chemical
propeties. Give reason. [4]
(b) Vanadium has the ability to exhibit various
oxidation states. How do you explain this?
(c) Complete and balance the
following equations.
(i) Fe2+ + Mn\(\mathrm{O}_4^{-}\) +
H+ →
(ii) Mn\(\mathrm{O}_4^{-}\) + H2O + I–
→
Answer:
Due to lanthanoid contraction, Zr and Hf possess nearly same
atomic and ionic radii, i.e. atomic radii are Zr = 160 pm and Hf = 159 pm and
ionic radii are Zr4+ = 79 pm and Hf4+ = 78 pm. Therefore,
these two elements show similar chemical and physical properties.
(b) Vanadium has the ability to exhibit wide range of oxidation states from +
2 to + 5 as it has 3d3 4s2 outer electronic configuration
and its standard
potentials are rather small due to stability of
V2+ as it has half-filled t2g level.
(c)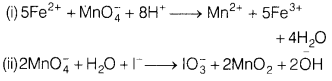
Question 30.
For a reaction, 2A + B → 2C following data were calculated
experimentally. The experiments were performed with varied concentration and for
each initial rate of formation of C was recorded. [4]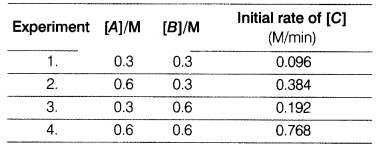
Answer the following questions on the basis of above data.
(a) Why were the experiments performed multiple times? Justify.
Answer:
Different sets of data help in avoiding error in data collection and
provide more accurate data for analysis.
(b) Calculate the order of the reaction.
Answer:
(c) (i) Write the rate law of the reaction.
(ii) Which experiment is the
fastest in term of +he data? Justify.
Answer:
(i) Rate law of the
reaction, R = k[A]2[B]1
(ii) Experiment 4 is the
fastest as in 1 minute, 0.768 molar concentration of [C] was formed.
Or
Calculate rate constant of the reaction. Justify it. [4]
Answer:
Rate
constant k.
From Exp. 1,
Rate = k[A]2[B]1
0.096 =
k (0.3)2 (0.3)1
k = \(\frac{0.096}{0.3 \times 0.3
\times 0.3}\) = \(\frac{96}{27}\)
= 3.55 m-2 min-1
Section
E
(The following questions are
long answer type and carry 5 marks each. All questions have an internal
choice.)
Question 31.
Attempt any five of the following. [5]
(a) Why is the
E°value for Mn3+ /Mn2+ couple is much more
positive than that for Fe3+ /Fe2+?
Answer:
Mn2+ compounds are more stable due to half-filled d-orbitals.
Fe2+ compounds are comparatively less stable as they have six
electrons in their orbitals.
So, they tend to lose one electron from
Fe2+ and get stable 3d 5 configuration in Fe3+.
Therefore, comparatively high positive value of E° for Mn3+
/Mn2+ indicates the stability of Mn2+(d5)
whereas comparatively low value for Fe3+ / Fe2+ indicates
the extra stability of Fe3+ (d5).
(b) Why transition metals form alloys?
Answer:
Atoms of transition
metal can easily take place in the crystal lattice of another metal in the
molten state and are miscible with each other and thus forms alloys.
(c) Why Eu2+ is a strong reducing agent?
Answer:
Eu2+ having electronic configuration
[Xe]4f75d16s0 is a strong reducing agent
because in the aqueous solution, it gets back to its most stable +3oxidation
state which has [Xe]4f76d06s0 stable electronic
configuration.
(d) Explain why transition elements acts as catalyst?
Answer:
Many transition metals and their compounds are used as
catalyst.
Their catalytic activity is due to their ability to adopt multiple oxidation states and to form complexes. Transition metals acts as catalyst because of their variable valencies sometimes form unstable intermediate compounds and provide a new path with lower activation energy for the reaction, e.g. V2O5 (contact process), finely divided iron (in Flaber’s process) and nickel (in catalytic hydrogenation).
(e) What is the lanthanoid contraction?
Answer:
Lanthanoid contraction
The overall decrease in atomic and ionic radii from lanthanum to lutetium, due
to the imperfect shielding of one electron by another in the same subshell is
known as lanthanoid contraction.
(f) Why Zn2+ salts are white, while Cu2+ salts are
coloured?
Answer:
Cu2+ salts are coloured because
Cu2+ ion has 3d94s0 valence shell configuration
with one unpaired electron and therefore, it is paramagnetic in nature.
Hence, Cu2+ ion undergoes d-d transition and forms coloured
salts.
In contrast to Cu2+, Zn2+ has all paired
electrons due to 3d104s0 valence shell configuration and
therefore, it does not undergo d-d transition. Flence, Zn2+ salts are
white.
(g) Why transition elements show variable oxidation states?
Answer:
ns
and (n -1 )d electrons of transition metal have very little difference in the
energies and hence both can participate in bonding, which results in variable
oxidation states.
When ns-electrons take part in bonding, they exhibit lower
oxidation states, whereas when (n – 1)d-electrons alongwith ns-electrons
participate in bonding, they exhibit variable oxidation states.
Question 32.
(a) Write Nernst equation for the following cell reaction,
[5]
Zn |Zn2+(ag) || Cu2+(ag) | Cu(s)
Answer:
Nernst equation
of given cell is
Ecell = E°cell – \(\frac{2.303 R 7}{2
F}\) =
log\(\frac{\left[\mathrm{Zn}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}\)
(b) Write the equations for the reactions taking place at the two electrodes
during the electrolysis of acidified copper sulphate solution with copper
electrodes.
Answer:
At anode, Cu → At cathode, Cu2+ +
2e–
At cathode, Cu2+ + 2e– → Cu
Here,
both copper electrodes are used.
(c) What is the effect of increase in concentration of zinc ions on the
electrode potential of zinc electrode for which \(E_{\mathrm{Zn}^{2+} /
\mathrm{Zn}}\) equals to – 0.76 V?
Answer:
According to Nernst
equation,![]()
Hence, electrode potential will increase with increase in
concentration of Zn+ ions.
Or
(a) The molar conductivity of a 1.5 M solution of an electrolyte is found to
be 138.9 S cm2/mol. What is the conductivity of the solution?
Answer:
Molar conductivity, Am = 138.9 S cm2 mol
Molarity = 1.5
M, Conductivity, \(\kappa\) = ?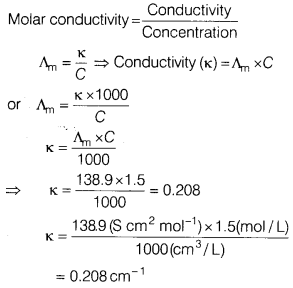
(b) What is the standard free energy change for the following reaction at
room temperature? Is the reaction spontaneous?
Zn(s) + Cu2+(ag) →
Zn2+(aq) + Cu(s)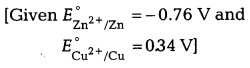
Answer: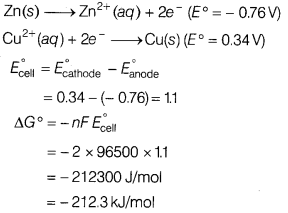
As the value of ∆G° is negative, so it is a spontaneous
reaction.
Question 33.
Compound A (C6H12O2) on
reduction with LiAlH4 yielded two compounds B and C. The compound B
on oxidation gave D which on treatment with aqueous alkali and subsequent heat
produces E. Also, the oxidation reaction of D gave monobasic acid (molecular
weight 60) and the catalytic hydrogenation of E gives the compound C. Identify
A, B, C, D and E. [5]
Answer:
The compound A which gives
CH3CH2OH on reduction with LiAIH4 will be
CH3COOC4H9 (n-butyl acetate).
Since, the
compound D has been obtained by oxidation of the compound B. Therefore, B is an
alcohol having formula CH3CH2OH.
The compound E is
monobasic acid having molecular weight 60. Therefore, it is acetic acid
CH3COOH.
The compound D which on oxidation gives acetic acid is
CH3CHO.
The reactions involved may be given as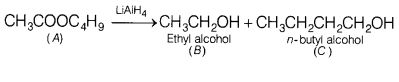
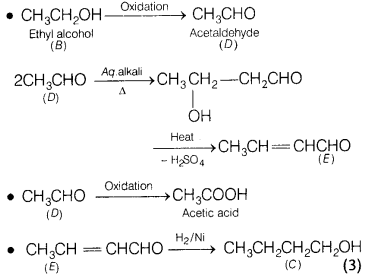
Or
(a) Give reasons of the following observations. [5]
(i) CH3CHO
is more reactive than CH3COCH3 towards reaction with
HCN.
Answer:
CH3CHO is more reaction than
CH3COCH3 towards reaction with HCN because of the fact
that due to smaller +I-effect of one alkyl group (—CH3) in
CH3CHO as compared to larger +I-effect of two alkyl
(—CH3)2 groups in CH3COCH3, the
magnitude of positive charge on the carbonyl carbon is more in CH3CHO
than in CH3COCH3.
Also, the steric effect is more
pronounced in case of CH3COCH3.
(ii) Cl—CH2COOH is a stronger acid than CH3COOH.
Answer:
CI —CH2COOH is a stronger acid than CH3COOH. It
is because —CI group exhibits -I-effect which makes the carboxylate ion more
stable.
Higher the stability of carboxylate ion, easier is the removal of
proton from the carboxylic acid and stronger is the acid. In CH3COOH,
—CH3 group has + I-effect which destabilises it. Hence,
CH3COOH is a weaker acid.
(iii) Aromatic carboxylic acids do not undergo Friedel-Crafts reaction.
Answer:
Due to the presence of strong —COOH group, aromatic carboxylic acids
do not undergo for Friedel-Crafts reaction.
(b) Arrange the following compounds in the increasing order of their property
as indicated.
(i) CH3COCH3,
C6H5COH3, CH3CHO (reactivity towards
nucleophilic addition reaction)
Answer:
The increasing order of their
reactivity towards nucleophilic addition reaction is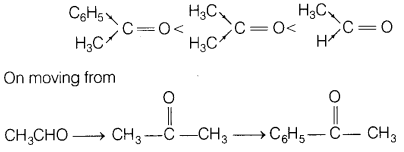
+I -effect, i.e. electron donating effect of alkyl group increases which increases the electron density on C-atom of carbonyl group, and inC6H5COCH3, phenyl group get resonance stabilised, making it stable.
Due to this reason it is less reactive towards nucleophilic addition as the
attack of nucleophile becomes lower.
Further, steric effects of methyl and
phenyl groups around carbonyl carbon atom makes the attack of nucleophile on
carbonyl carbon difficult.
(ii) ClCH2COOH, FCH2COOH, CH3COOH (acidic
character)
Answer:
The correct increasing order of their acidic character
is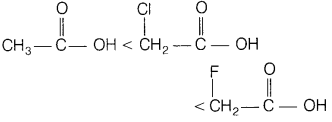
F-being more electronegative element produces greater-I-effect than Cl-atom due
to which F-atom withdraw electrons from O—H bond and thereby making O—H bond
weaker and hence, facilitates the release of H+ ion from O—H
bond.
Hence, FCH2COOH is stronger acid than CICH2COOH and CH3COOH.
In CH3COOH, due to +I-effect of methyl group, electron density in O—H bond increases. As a result, release of H+ ions from acetic acid becomes more difficult.