CBSE Sample Papers for Class 12 Chemistry Set-5
Class 12thCBSE Sample Papers for Class 12 Chemistry Set-5
CBSE Sample Papers for Class 12 Chemistry Set 5 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section
A
(The following questions are
multiple-choice questions with one correct answer. Each question carries 1 mark.
There is no internal choice in this section.)
Question 1.
For a cell reaction involving two electrons change the
standard emf of the cell is found to be 0.295 V at 25°C. The equilibrium
constant for the reaction at 25°C will be [1]
(a) 2.95 × 102
(b) 10
(c) 1 × 1010
(d) 1 × 10-10
Answer:
(c)
1 × 1010
Using E°cell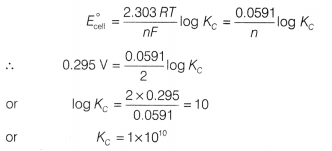
Question 2.
The compound which does not reduce Fehling solution is [1]
(a) CH3COOH
(b) HCOOH
(c) HCHO
(d) CH3COH
Answer:
(a) CH3COOH
Those compounds which have —CHO group can reduce Fehling solution. Thus,
CH3COOH does not react with Fehling solution.
Moreover HCOOH have
—CHO group present in it. Thus, it also reduces Fehling solution.
Question 3.
Pair of compounds, which give positive test with Tollen’s
reagent is [1]
(a) glucose and sucrose
(b) fructose and glucose
(c)
glucose and fructose
(d) All of the above
Answer:
(c) glucose and
fructose
Because of presence of aldehyde group, glucose reduces Tollen’s reagent. Although fructose contain ketonic group, but in the presence of base, it(fructose) again rearranges into glucose, due to which it also reduces Tollen’s reagent.
Question 4.
Alcohol vapours are passed over which of the following
catalysts to give aldehydes and ketones? [1]
(a) S or Pd
(b) Ag or Cu
(c) F or Cl
(d) Li or K
Answer:
(b) Ag or Cu
Alcohol vapours are passed over heavy metal catalysts (Ag or Cu) to give aldehydes and ketones. Primary and secondary alcohols gives aldehydes and ketones respectively.
Question 5.
Halogenation, sulphonation, Friedel-Crafts reaction of
haloarene comes under [1]
(a) nucleophilic substitution reaction of
benzene
(b) electrophilic substitution reaction of benzene
(c) addition
reaction of benzene
(d) elimination reaction of benzene
Answer:
(b)
electrophilic substitution reaction of benzene
Haloarenes undergo electrophilic substitution reactions of benzene ring such as halogenation, nitration, sulphonation and Friedel-Crafts reaction.
Question 6.
Lanthanides form complex with following ligand. [1]
(a)
F–
(b) B–
(c) Cl–
(d)
I–
Answer:
(a) F–
F– have small size and high electronegativity, thus it forms complex with lanthanides easily.
Question 7.
The decomposition of ammonia gas on a hot platinum surface is
[1]
(a) zero order reaction
(b) first order reaction
(c) second order
reaction
(d) third order reaction
Answer:
(a) F–
The decomposition of NH3 gas on a hot platinum surface is zero
order reaction because the rate of this reaction is independent on the
concentration of ammonia.![]()
Rate = k[NH3]°
Hence, this reaction is a
zero-order reaction.
Question 8.
Which of the following statements is not correct for amines?
[1]
(a) Aniline and other arylamines are usually colourless but get coloured
on storage due to atmospheric oxidation.
(b) Lower aliphatic amines are
gases.
(c) Primary amine gives monoalkyl sulphonamide with Hinsberg test.
(d) When aniline reacts with sulphuric acid, m-aminobenzene sulphonic acid is
formed.
Answer:
(d) When aniline reacts with sulphuric acid,
m-aminobenzene sulphonic acid is formed.
Sulphonation of aniline is done by fuming H2SO4 at
453-473 K to get p-aminobenzene sulphonic acid (sulphanilic acid).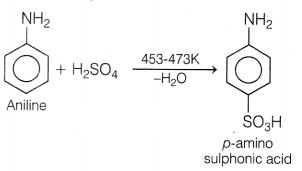
Question 9.
The major product of the acid catalysed of 3, 3-dimethylbutene
is [1]
(a) 2-methylbutanol
(b) 2, 3-dimethylbutan-2-ol
(c) 3,
3-dimethylbutan-2-ol
(d) 2, 2-dimethylbutanol
Answer:
(b) 2,
3-dimethylbutan-2-ol
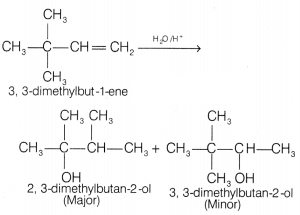
Question 10.
For the reaction, A + 2B → C,
Rate = k[A][B]
If the
concentration of reactant B is doubled, keeping the concentration of A constant,
the value of rate constant will be [1]
(a) decreased by two times
(b)
increased by four times
(c) increased by two times
(d) no change
Answer:
(c) increased by two times
Rate law for the given reaction,
A + B → C
Rate = k[A] [B]
Rate of
reaction with respect to B is of first order
R1 = k[A][B]
When
the concentration of reactant ‘B’ is doubled, then R2 = k[A][2B]
=
2k[A][B] = 2R1
∴ As concentration of reactant ‘B ’ is doubled
keeping the concentration of A constant, rate of reaction doubles.
Question 11.
Identify the major product of the following reactions?
[1]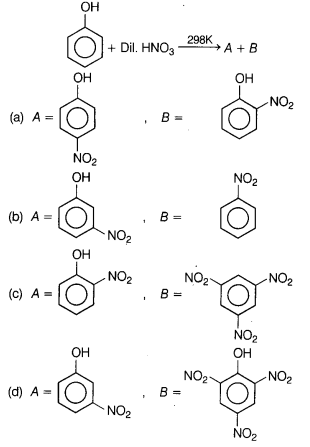
Answer:
(a)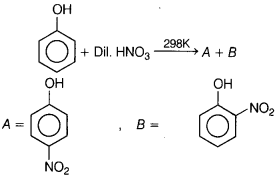
Nitration of phenol with dilute nitric acid at low temperature (298 K) yield
a mixture of ortho and para-nitrophenols.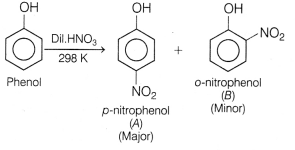
Question 12.
The trend of which property is represented by the following
graph? [1]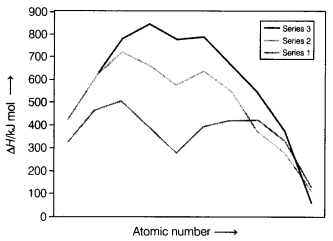
(a) Ionisation enthalpy
(b) Atomic radii
(c) Enthalpy
of atomisation
(d) Melting point
Answer:
(c) Enthalpy of
atomisation
The given graph represents the enthalpy of atomisation. The metals of the second and third series have greater enthalpies of atomisation than the corresponding elements of the first series. The maxima at about the middle of each series indicate that one unpaired electron per d-orbital is particularly favourable for strong interatomic interaction.
Direction (Q. Nos. 13-16) In the following questions an Assertion (A) is
followed by a corresponding Reason (R). Use the following keys to choose the
appropriate answer. .
(a) Both (A) and (R) are true, (R) is the correct
explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct
explanation of (A).
(c) (A) is true, (R) is false.
(d) (A) is false, (R)
is true.
Question 13.
Assertion (A) Di-fert-butyl ether cannot be prepared by
Williamson’s synthesis.
Reason (R) Tert-butyl bromide on treatment with
sodium fert-butoxide preferentially undergoes elimination from iso-butylene and
tert-butyl alcohol. [1]
Answer:
(a) Both (A) and (R) are true, (R) is the
correct explanation of (A).
Question 14.
Assertion (A) As a lead storage battery gets discharged,
density of electrolyte present in it decreases.
Reason (R) Lead and lead
dioxide both react with sulphuric acid to form lead sulphate. [1]
Answer:
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
Sulphate is consumed from electrolyte due to formation of lead sulphate, thus density decreases.
Question 15.
Assertion (A) In the presence of enzyme, substrate molecule
can be attacked by the reagent effectively.
Reason (R) Active sites of
enzymes hold the substrate molecule in a suitable position. [1]
Answer:
(a) Both (A) and (R) are true, (R) is the correct explanation of (A).
Question 16.
Assertion (A) Oxidation of ketones is easier than
aldehydes.
Reason (R) C—C bond of ketones is stronger than C—H bond of
aldehydes. [1]
Answer:
(d) (A) is false, (R) is true.
Oxidation of ketones is rather difficult than aldehydes because C—C bond of ketones is stronger than C—H bond of aldehydes. Therefore, ketones cannot be oxidised by weak oxidising agent.
Section
B
(This section contains 5
questions with internal choice In one question. The following questions are very
short answer type and carry 2 marks each.)
Question 17.
For a reaction, the rate law expression is represented as
follows
Rate = k[A] [B]1/2
(a) Interpret whether the reaction
is elementary on complex. Give reason to support your answer. [2]
Answer:
For an elementary reaction, order of reaction must be equal to the molecularity
but molecularity should be integral.
For the given rate law, order of
reaction comes out to be \(\frac{3}{2}\). As molecularity cannot be fractional,
therefore, order is not equal to molecularity. Hence, the given rate law do not
belong to an elementary
reaction.
(b) Write the units of rate constant for this reaction, if the concentration
of A and B is expressed in moles/L
Answer:
Units of rate constant are
mol1/2L1/2s-1
Question 18.
Calculate the mass of compound (molar mass = 256 g
mol-1) to be dissolved in 75 g of benzene to lower its freezing point
by 0.48 K(Kf = 51.2 K kg mol-1). [2]
Answer:
Given
that, Kf = 5.12 K kg mol-1, W1 = 75
g,
∆Tf = 0.48K, M2 = 256 g
mol-1
∆Tf = \(\frac{K_f \times W_2 \times 1000}{W_1
\times M_2}\)
or 0.48 = \(\frac{5.12 \times W_2 \times 1000}{75 \times
256}\)
∴ w2 = \(\frac{0.48 \times 75 \times 256}{5.12 \times
1000}\) = 1.8 g
Question 19.
Give reason for the following.
(a) The C—Cl bond length in
chlorobenzene is shorter than in CH3 —Cl. [2]
Answer:
Due to
delocalisation of Ione pairs of electrons of the X atom over the benzene ring,
C—X bond in halobenzene acquires some double bond character while in
CH3—X, C—X bond is a pure single bond. Therefore, C—X bond in
halobenzene is shorter than in CH3—X.
(b) Name the product(s) formed when 2-bromopropane undergoes
dehydrohalogenation reaction.
Answer: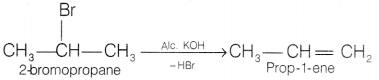
In this reaction, 2-bromopropane undergoes
dehydrohalogenation by the loss of HBr. This reaction is an example of
elimination reaction.
Question 20.
(a) Give simple tests to distinguish between pentan-2-one and
pentan-3-one. [2]
Answer:
Propan-2-one and pentan-3-one can be
distinguished by iodoform test.
On heating with NaOH +I2 or
[NaOl], propan-2-one being a methyl ketone forms yellow ppt. of iodoform,
whereas pentan-3-one does not.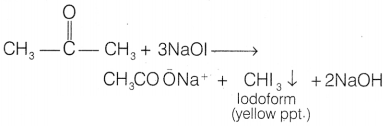
(b) Illustrate the Clemmensen reduction reaction giving suitable example.
[2]
Answer:
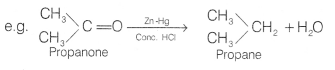
Clemmensen reduction The carbonyl group of aldehydes and
ketones is reduced to — CH2 group on treatment with zinc-amalgam and
concentrated hydrochloric acid. This reaction is known as Clemmensen
reduction.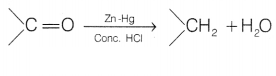
Or
How are the following conversions carried out? [2]
(a) Propene to
propan-2-ol
Answer: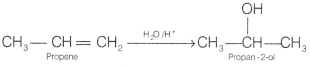
(b) Ethyl chloride to ethanal
Answer: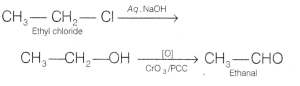
Question 21.
(a) How hormones and vitamins differ with respect to their
sources and functions? [2]
Answer:
| Vitamins | Hormones |
| Sources Organic compounds which cannot be produced by body must be supplied in small amounts through food. | Hormones are the chemical messengers produced by ductless gand |
| Function It perform specific biological functions for normal maintenance of optimum growth and health of the organism. | Function It provide information to distant organs. |
(b) Describe the primary structure of proteins.
Answer:
Primary
structure In a protein molecule, one or more polypeptide chains may be present.
Each polypeptide chain in a protein is linked together in a specific sequence of
amino acids, this sequence of amino acids is termed as primary structure of
proteins.
Any change in primary structure, i.e. in the sequence of amino acid
generates a new protein.
Section
C
(This section contains 7
questions with internal choice in one question. The following questions are
short answer type and carry 3 marks each.)
Question 22.
Using valence bond theory, explain the following in relation
to the complex [CoCl4]2-.
(a) Type of
hybridisation.
(b) Type of complex-(inner or outer orbital).
(c) Nature of
complex-(diamagnetic or paramagnetic). [3]
Answer:
In
[CoCl4]2-, Co is in +2 state and has an outer electronic
configuration of 3d7.
Cl– is a weak field ligand so,
pairing of electrons will not occur.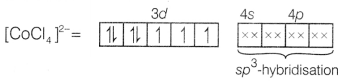
Four pairs of electrons one from each Cl– ion
occupy the four sp3-hybrid orbitals.
Therefore, the complex has
tetrahedral geometry and paramagnetic nature as it contains three unpaired
electrons.
Thus, the hybridisation is sp3, i.e. the complex is
outer orbital complex.
Question 23.
(a) Write an expression that relates the molar conductivity
of a weak electrolyte to its degree of dissociation. [3]
Answer:
The
degree of dissociation is the ratio of molar conductivity (Λm) at a
specific concentration C to the molar conductivity at infinite dilution, i.e.
limiting molar conductivity, Λ°m, and express as,
∴ α =
\(\frac{\Lambda_{\mathrm{m}}}{\Lambda_{\mathrm{m}}^{\circ}}\)
(b) State Faraday’s first law of electrolysis.
Answer:
Faraday’s first
law of electrolysis The amount of substance deposited during electrolysis is
directly proportional to quantity of electricity passed.
m ∝ Q, m ∝ f or m =
Zlt,
where, Z is electrochemical equivalent, I is current in ampere, t is
time in seconds and Q is charge in coulomb.
(c) How much charge in terms of Faraday is required for the reduction of 1
mole of Cu2+ to Cu?
Answer:
Charge required for the reduction
of 1 mole of Cu2+ to Cu is 2F.
Question 24.
Write the IUPAC name, name of the equation and chemical
equation for the following reactions. [3]
(a) When phenol treated with CHCl3 in the presence of sodium
hydroxide.
Answer: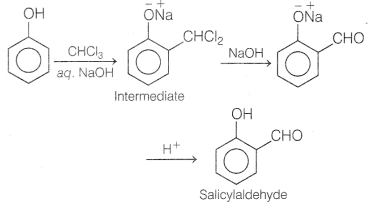
This reaction is known as Reimer-Tiemann reaction.
(b) Phenol reacts with NaOH and CO2.
Answer:
When phenol is
heated with NaOH, then with CO2 and water, ortho-hydroxy benzoic acid
is formed as ‘ the main product.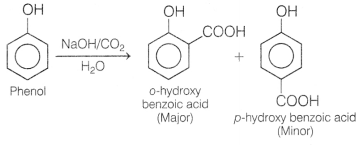
This reaction is known as Kolbe’s reaction.
Question 25.
A, B and C are three non-cyclic functional isomers of a
carbonyl compound with molecular formula C4H8O. Isomers A
and C gives positive Tollen’s test whereas isomer B does not give Tollens’s test
but gives positive iodoform test.
Isomers A and B on reduction with
Zn-Hg/conc.HCl give the same product D. Identify A, B, C and D. [3]
Answer:
- Tollen’s test is given by aldehydes and not by ketones.
- Iodoform test is given by compounds that have a methyl group attached to a
carboxyl carbon
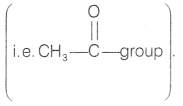
- The reagent which is used to reduce carbonyl carbon into methylene is Zn-Hg/conc. HCI which is Clemmensen’s reagent.
So, compound A is an aldehyde with molecular formula
C4H8O and structure will be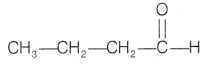
Compound B does not give Tollen’s test but gives the Iodoform
test. So, compound B is methyl ketone with molecular formula
C4H8O.
Thus, the compound is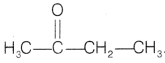
Compound B and A gave the same product D on reacting with
Clemmensen reagent. So, the possible structure of D is
H3C—CH2—CH2—CH3.
Compound C is
also an aldehyde as it gives positive Tollen’s test. So, the aldehyde may
be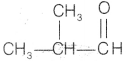
as it is an another possible aldehyde isomer of
C4H8O.
Or
An organic compound with molecular formula C9H10O,
forms 2,4-DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro
reaction. On vigorous oxidation, it gives 1,2-benzene dicarboxylic acid.
Identify the compound and write down the reaction involved Tollen’s reagent.
Answer:
As the given compound with molecular formula
C9H10O, forms a 2,4-DNP derivative and reduces Tollen’s
reagent, thus it must be an aldehyde.
On vigorous oxidation, it gives
1,2-benzene dicarboxylic acid and also undergoes Cannizzaro reaction, hence —CHO
group is directly attached to the benzene ring.
Therefore, it must be an ortho-substituted benzaldehyde and the only
o-substituted aromatic aldehyde which have C9H10O
molecular formula is o-ethyl benzaldehyde.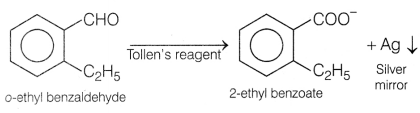
Question 26.
(a) Name the type of structure of proteins represented in
diagram. [3]
Answer:
Proteins are found to exist in two different types of
structures viz. α-helix and β-pleated sheet structure, The given structure
represents a-helix structure.
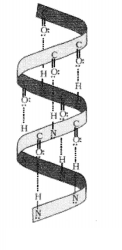
(b) Name the type of bond present between the pair of bases of the
polynucliotide chains of the double helix.
Answer:
Hydrogen bonding
present between the —NH — group of each amino acid residue and the![]()
of an adjacent turn of the helix mainly helps in stabilising
the a-helix structure of proteins.
(c) Write one difference between α-helix and β-pleated sheet structures of
protein.
Answer:
In α-helix structure of proteins, the polypeptide chains
are stabilised by intramolecular hydrogen bonding whereas β-pleated sheet
structure of proteins is stabilised by intermolecular hydrogen bonding.
Question 27.
(a) Name the major product formed when chlorobenzene is
treated with CH3COCl in the presence of anhydrous AlCl3.
Write the reaction involved. [3]
Answer: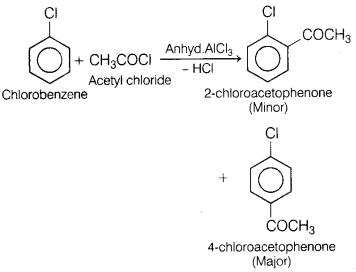
This reaction is an example of Friedel-Crafts acylation in
which chlorobenzene is reacted with acetyl chloride in the presence of anhyd.
AlCl3 and three products are formed in which 4-chloroacetophenone is
the major product.
(b) Why Grignard reagent should be prepared under anhydrous conditions?
Answer:
Grignard reagents are very reactive.
In the presence of moisture,
they react to give alkanes.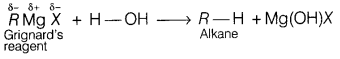
Therefore, Grignard reagents should be prepared under
anhydrous conditions.
Question 28.
The rate of a first order reaction is 0.04 mol L-1
s-1 at 10 min and 0.03 mol L-1 s-1 at 20 min
after initiation. Find the half-life of a reaction. [3]
Answer:
For a
first order reaction, A → Products,
For concentration of the reactant at two
different times, the rate constant is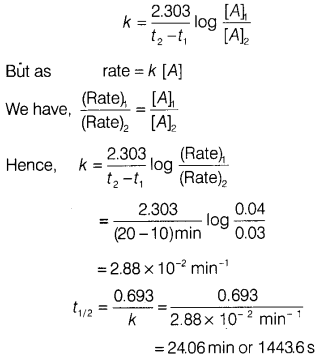
Section
D
(The following questions are
case-based questions. Each question has an internal choice and carries 4(1+1+2)
marks each. Read the passage carefully and answer the questions that
follow.)
Question 29.
The cell constant is usually determined by measuring the
resistance of the cell containing a solution whose conductivity is already
known. For this purpose, we generally use KCl solutions whose conductivity” is
known accurately at various concentrations at 298 K temperature as mentioned in
given table. [4]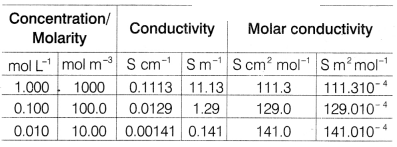
Answer the following questions
(a) Express the relation for the conductivity of a solution in the cell, the
cell constant and the resistance of solution in the cell.
Answer:
Conductivity,
\(\kappa\) = \(\frac{1}{R}\) × \(\frac{1}{A}\)
where
\(\kappa\) = conductivity,
R = resistance
and \(\frac{I}{A}\) = cell
constant
Or
How does the conductivity of solutions of different electrolytes in the same
solvent and at a given temperature differs ?
Answer:
The conductivity of
solutions of different electrolytes in the same solvent at a given temperature
differs due to charge and size of the ions in which they dissociate, the
concentration of the ions or ease with which the ions move under a potential
gradient.
(b) Why does the conductivity of the solution decreases with dilution?
Answer:
On dilution, the number of ions per unit volume decreases. Hence, the
conductivity decreases.
(c) Explain with the graph, the variation of molar conductivity of KCl with
dilution with respect to given table.
Answer:
For strong electrolyte like
KCl, Λm increases slowly with dilution.
It is found to vary with
concentration according to the Debye-Huckel-Onsager equation.![]()
where, A and B are called Debye-Huckel constants. If solution
is diluted (concentration is decreased), there is decrease in ionic attractions
hence, molar conductivity increases with decrease in concentration.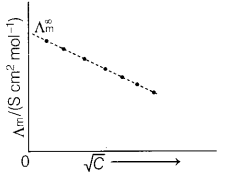
Question 30.
Lewis’ formulation of the nature of the chemical bond is in
many ways the precursor of valence bond theory. Lewis’ paper, “The Atom and The
Molecule”, contains plenty of ideas, some of which were later embedded into
valence bond theory. His electron-pair model and its dynamic nature regarding
polarity, was formulated 11 years before the onset of valence bond theory” in
physics.
Valence bond theory describes covalent bond formation as well as the electronic structure of molecules. The theory assumes that electrons occupy atomic orbitals of individual atoms within a molecule, and that the electrons of one atom are attracted to the nucleus of another atom.
Pauling and Slater subsequently developed the creative notion of hybridisation, which forms localised bonds, which ‘‘determine” the molecular geometry. The angles of these hybrids follow the observed bond angles. Thus, for example, sp is the hybridisation for co-linear bonds, sp2 for three trigonal bonds, sp3 for tetrahedral bonds, while sp3d and sp3d2 are for bonds directed to the corners of a trigonal bipyramid and octahedron, respectively. As such, these hybridisations allowed a modern discussion of molecular geometries in a chemistry-teaching tools that allow young students to figure out the geometry of molecules, and in some crude way, also their bonding.
Answer the following questions. [4]
(a) Why s-orbital does not show preference to any direction?
Answer:
The s-orbtial is spherically symmetric in shape, so it does not show preference
to any direction. It is the same from all the directions.
(b) Using valence bond theory, predict the hybridisation of
[CoF6]3-.
Answer:
[CoF6]3-
Here, CO is present in +3 oxidation state.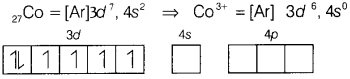
F being a weak ligand is unable to pair up its unpaired electrons thus,
occupy 4s, 4p, and 4d empty orbitals as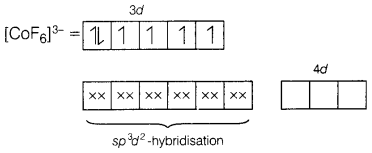
(c) Can valence bond theory predict the magnetic nature of a complex? If yes,
justify your answer with example.
Answer:
The VBT only provides us
qualitative magnetic nature of the complex.
The magnetic properties of
transition metals can be predicted by the number of unpaired electrons present
by filling of electron in an orbital and hence, can predict the magnetic
property whether diamagnetic or paramagnetic.
e.g. In [NiCl4]2-, the central metal ion present in
this complex is Ni2+ and electronic configuration is Ni2+
is [Ar]3d84s0.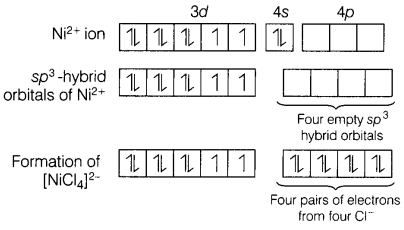
Two unpaired electrons are present. Hence, the complex ion is
paramagnetic.
Or
Imagine you have provided with two following complexes. Explain which of these complexes have square planar structure? [4]
(i) [Ni(CN)4]2-
Answer: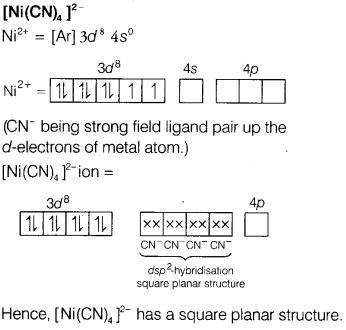
(ii) K4[Mn(CN)6]
Answer:
In [Ni(CO)4],
Ni has zero oxidation state.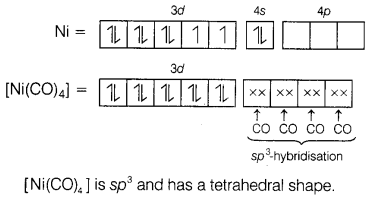
Section
E
(The following questions are
long answer type and carry 5 marks each. All questions have an internal
choice.)
Question 31.
Attempt any five of the following. [5]
(a) Explain the nature of bonding in La2O3 and
Lu2O3.
Answer:
As the size decreases covalent
character increases, i.e. on moving from La to Lu. Therefore,
La2O3 is more ionic and Lu2O3 is
more covalent.
(b) What is the trend in the stability of oxo salts of lanthanoids from La to
Lu.?
Answer:
The oxo salts of lanthanoids contains lanthanoid element as
cation and oxygen as anion. According to Fajan’s rule, smaller is the size of
cation greater will be its polarization power. Since, the size decreases from La
to Lu, polarizing power of cation increases and accordingly distort the electron
cloud more effectively on anion. Due to this, the bond strength decreases and
stability will also decrease.
(c) Actinoid contraction is greater from element to element than lanthanoid
contraction. Why?
Answer:
The decrease in atomic (or ionic) radii
(actinoid contraction) in actinoids is greater than lanthanoid contraction
because 5f-electrons have poor shielding effect as compared to 4f-electrons.
Therefore, the effect of increased nuclear charge leading to contraction in
size, is more in case of actinoids.
(d) Why is the radii of 4d and 5d-block elements is almost same?
Answer:
The f-orbitals have very poor shielding effect. Thus, in the case of
5d-block elements the nuclear charge increase on valence shell electrons and
result of which size of these elements decrease to a great extent. This
phenomenon is known as lanthanoid contraction. Thus, radii of 4d and 5d block
elements are almost similar.
(e) What is the trend in acidic character of lanthanoid oxides?
Answer:
Acidic character of oxides increases from La to Lu. As the size of La
to Lu decreases, the ability to loose an electron decreases. Hence, acidity will
increase.
(f) Complete the following equation, Mn\(\mathrm{O}_4^{-}\) + 8H+
+ 5e– →
Answer:
Mn\(\mathrm{O}_4^{-}\) + 8H+ +
5e– → Mn2+ + 4H2O
(g) Out of Cr3+ and Mn2+, which is a stronger oxidising
agent and why?
Answer:
Mn3+ is stronger oxidising agent because
the change from Mn3+ to Mn2+ results in half-filled
(d5) configuration which has extra stability. Thus, it behaves as a
strong oxidising agent. On the other hand, Cr3+ has d3
configuration, i.e. half-filled t2g level, so, it is already stable
and does not reduce to less stable Cr2+.
Question 32.
(a) On mixing two liquids X and Y, volume of the resulting
solution decreases. What type of deviation from Raoult’s law is shown by the
resulting solution? [5]
What change in temperature would you observe after
mixing liquids X and Y?
Answer:
On mixing two liquids X and Y, the
decrease in volume of the resulting solution suggests that a non-ideal solution
is formed which shows negative deviation from Raoult’s law.
In case of the
solutions showing negative deviations from Raoult’s law, ∆H mixing (i.e.
enthalpy of mixture) is negative. Thus, on mixing X and Y liquids, evolution of
heat takes place, i.e. the temperature of the resulting solution increases.
(b) What happens when we place the blood cell in water (hypertonic solution)?
Give reason.
Answer:
When we placed the blood cell in hypertonic solution
then due to osmosis, water will flow out of the cell and it would shrink.
(c) A 0.561 m solution of unknown electrolyte depresses the freezing point of
water by 2.93°C. What is van’t Hoff factor for this electrolyte?
The freezing
point depression constant (Kf) for water is 1.86°C kg
mol-1.
Answer:
Given, m = 0.561 m, ∆T<sub.f = 2.93°C and
Kf = 1.86° C kg mol-1
∆Tf =
iKfm
i = \(\frac{\Delta T_t}{K_t m}\)
= \(\frac{2.93^{\circ}
\mathrm{C}}{1.86^{\circ} \mathrm{C} \mathrm{kg} \mathrm{mol}^{-1} \times 0.561
\mathrm{~m}}\) = 2.807
Or
(a) Which type of deviation shown by the mixture of chloroform and acetone?
What is the reason behind the deviations shown by different solutions or
mixtures? [5]
Answer:
The mixture of chloroform and acetone, shows
negative deviation from Raoult’s law.
The positive and negative deviations
from Raoult’s law are based on interactions between the component of the
mixture.
In case of positive deviation, the solute and solvent interactions are weaker than those of solute-solute or solvent-solvent interactions, while in case of negative deviation the interactions of solute-solvent are stronger than those of solute-solute or solvent-solvent.
(b) The vapour pressure of pure ethanol and acetone are 450 mm and 700 mm of
Hg respectively at 350 K. Find out the composition of the liquid mixture if
total vapour pressure is 600 mm of Hg. Also, find the composition in the vapour
phase.
Answer:
Given : Vapour pressure of pure ethanal (pA)
= 450 mm of
Hg
Vapour pressure of pure acetone (p°B)
= 700 mm of Hg ;
ptotal = 600 mm of Hg; χA = ?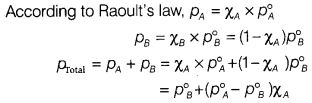
On substituting the given values into the above equation, we
get
600 = 700 + (450 – 700 )χA
100 = 250χA ⇒
χA = \(\frac{100}{250}\) = 0.40
Thus, the composition of the
liquid mixture will be
χA = 0.40 and χB = 1 – 0.40 =
0.60
Calculation of composition in the vapour phase,
PA =
χA × P°A = 0.40 × 450 mm of Hg = 180 mm of Hg
PB =
χB × P°B = 0.60 × 700 mm of Hg = 420 mm of Hg
Mole
fraction of A in the vapour phase
= \(\frac{p_A}{p_A+p_B}\) =
\(\frac{180}{180+420}\) = 0.30
Mole fraction of B in the vapour phase
= 1
– 0.30 = 0.70
Question 33.
A hydrocarbon ‘A’, (C4H8) on reaction
with HCl gives a compound ‘B’, (C4H9Cl), which on reaction
with 1 mole of NH3 gives compound ‘C’,
(C4H11N). On reacting with NaNO2 and HCl
followed by treatment with water, compound ‘C yields optically active alcohol,
‘D’. Ozonolysis of A’ gives 2 moles of acetaldehyde. Identify compounds A to
‘D’. Explain the reactions involved. [5]
Answer:
On reaction with HCI,
hydrocarbon ‘A’ gives ‘B’ which is haloalkane, that means A is an
alkene.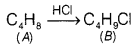
On reacting with NH3, \(\mathrm{NH}_2-\)
substitutes Cl in compound ‘B’ to give ‘C’.![]()
‘C gives a diazonium salt with NaNO2/HCl that
liberates N2 to give optically active alcohol, that means that ‘C’ is
an aliphatic amine.
Since, the number of carbon atoms in amine is the same as
in compound A’, products of ozonolysis of compound ‘A’ are two molecules of
CH3—CH=O.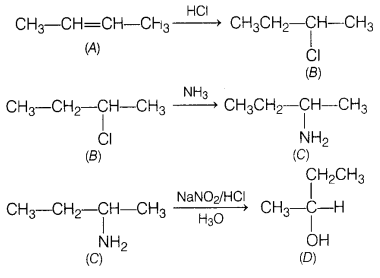
Or
(a) Give reasons of the following observations.
(i) Methylamine in water
reacts with ferric chloride to precipitate hydrated ferric oxide.
Answer:
Methylamine being more basic than water, accepts a proton from water and
OH– ions are produced which further reacts with ferric ion to give
brown ppt. of hydrated ferric oxide.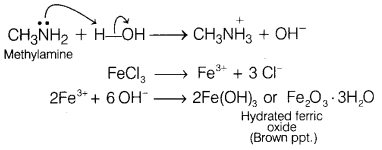
(ii) Aniline does not undergo Friedel-Crafts reaction.
Answer:
Aniline
does not undergo Friedel-Crafts reaction because the reagent AlCl3
being electron deficient acts as a Lewis acid and attacks on the lone pair of
nitrogen present in aniline to form insoluble precipitate and reaction does not
proceeds.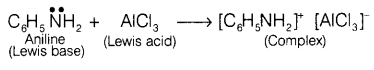
(iii) Diazonium salts of aromatic amines are more stable than those of
aliphatic amines.
Answer:
Diazonium salts of aromatic amines are more
stable than those of aliphatic amines because these are resonance stabilised
while no such resonance stabilisation is possible in the corresponding diazonium
salts of aliphatic amines.
(b) Arrange the following compounds in
(i) the increasing order of dipole
moment. CH3CH2CH3,
CH3CH2NH4, CH3CH2OH
Answer:
CH3CH2CH3 <
CH3CH2NH2 <
CH3CH2OH
(ii) the increasing order of basic characteristics.
NH3,
(CH3)3 N, CH3 NH2,
(CH3)2 NH
Answer:
(CH3)2NH
> CH3NH2 > (CH3)3N >
NH3