CBSE Sample Papers for Class 12 Chemistry Set-2
Class 12thCBSE Sample Papers for Class 12 Chemistry Set-2
CBSE Sample Papers for Class 12 Chemistry Set 2 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section
A
(The following questions are
multiple-choice questions with one correct answer. Each question carries 1 mark.
There is no internal choice in this section.)
Question 1.
For d4 ions, the fourth electron enters one of the
eg-orbitals giving the configuration \(t_{2 g}^3 e_g^1\), when
[1]
(a) ∆0 > P
(b) ∆0 < P
(c) ∆0
= P
(d) ∆0 ≥ P
Answer:
(b) ∆0 < P
The fourth electron enters one of theeg-orbitals giving the configuration \(t_{2 g}^3 e_g^1\) when ∆0 < P.
Question 2.
The product formed during Hell-Volhard-Zelinsky reaction is
[1]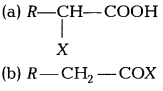
Answer:
(a)
Carboxylic acids having an a-hydrogen are halogenated at the
a-position on treatment with chlorine or bromine in the presence of small amount
of red phosphorus to give a-halocarboxylic acids. This reaction is known as
Hell-Volhard-Zelinsky reaction.
Question 3.
The pair of bases in DNA are held together by [1]
(a) ionic
bonds
(b) H-bond
(c) phosphate group
(d) deoxyribose group
Answer:
(b) H-bond
The pair of bases in DNA are held with each other by
H-bonds.
Question 4.
Which of the following is the most polar compound? [1]
(a)
Propanone
(b) Formaldehyde
(c) Propanal
(d) Hexan-3-one
Answer:
(b) Formaldehyde
Among the given compounds, formaldehyde is the most polar
due to lowest electron density on C of carboxyl group, whereas in all other
cases, the electron density on C is high due to +I effect of alkyl groups.
Question 5.
Identify A B and C in the given sequence of reactions,
[1]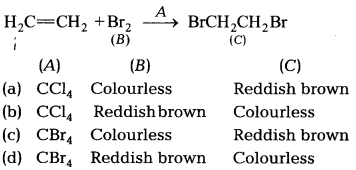
Answer:
(b) Addition of bromine in Ccl4 to an alkene results in
discharge of reddish brown colour of bromine. It is an important method for
detection of double bond in a molecule.
The electrophilic addition results in
the synthesis of vic-dibromictes, which are colourless.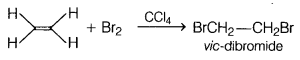
Question 6.
Match the properties with the elements of 3d-series:
[1]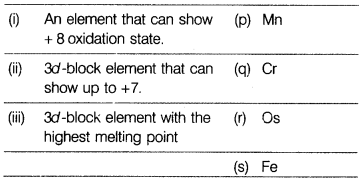
(a) (i) – (r), (ii) – (p), (iii) – (q)
(b) (i) – (r), (ii) – (s), (iii) –
(p)
(c) (i) – (p), (ii) – (q), (iii) – (r)
(d) (i) – (s), (ii) – (r),
(iii) – (p)
Answer:
(a) (i) – (r), (ii) – (p), (iii) – (q)
(i)
Osmium(Os) has an oxidation state starting from +2 to +8.
(ii) Mn [Ar]
3d54s2 shows +2, +3, +4, + 5, +6 and +7 oxidation state,
maximum number in 3d-series.
(iii) Chromium(Cr) has the highest melting point
due to its half-filled stable d-orbital and the unpaired electrons form strong
intermetallic bonds which are difficult to break immediately.
Question 7.
For a hypothetical reaction, R → P; Rate = -k[R]. The negative
sign used in the rate expression indicates [1]
(a) decrease in the
concentration of reactant with time.
(b) decrease in the concentration of
product with time.
(c) reaction is reversible.
(d) decrease in the rate
with time.
Answer:
(a) decrease in the concentration of reactant with
time.
For the given reaction, the negative sign indicates the decrease in
concentration of reactants with time.
Question 8.
Which of the following is not correct for amines? [1]
(a)
Ethyl methylamine cannot be prepared by Gabriel phthalimide synthesis.
(b)
N-ethylbenzene sulphonamide is insoluble in alkali.
(c) Amines are less
volatile than hydrocarbons.
(d) Amides can be converted into amines with same
number of carbon atoms by LiAlH4 /ether.
Answer:
(b)
N-ethylbenzene sulphonamide is insoluble in alkali.
N-ethylbenzene
sulphonamide is soluble in alkali because hydrogen attached to nitrogen in
sulphonamide is strongly acidic and forms a salt during reaction between these
two.
Question 9.
Hydroboration oxidation of 4-methyloctene would give [1]
(a) 4-methyloctanol
(b) 2-methyldecane
(c) 4-methylheptanol
(d)
4-methyl-2-octanone
Answer:
(a) 4-methyloctanol
Terminal alkenes react
rapidly with diborane to form primary trialkyl boranes which on oxidation gives
primary alcohols.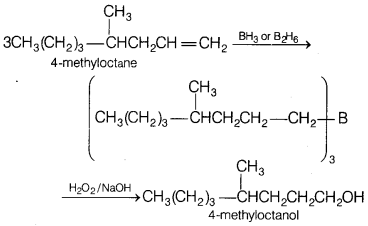
(In general, hydroboration oxidation involves the addition of water according to
anti-Markownikoff’s rule).
Question 10.
The half-life period of a first order reaction is 4 minutes,
the time after which 99.9% reaction gets completed is [1]
(a) 16 min
(b) 8
min
(c) 32 min
(d) 40 min
Answer:
(d) 40 min
Given,
t1/2 = 4min and a = 100; a – x = 0.1
Now, t1/2 =
\(\frac{0.693}{k}\) [For first order reaction]
∴ k = \(\frac{0.693}{4}\) ……
(i)
As we know that, k = \(\frac{2.303}{t}\)log\(\frac{a}{a-x}\)
t =
\(\frac{2.303 \times 4}{0.693}\)log\(\frac{100}{0.1}\) [From Eq.(i)]
t =
39.88 min ≈ 40 min
Question 11.
Boiling point of alcohol is comparatively higher than that of
corresponding alkane due to [1]
(a) intermolecular hydrogen bonding
(b)
intramolecular hydrogen bonding
(c) volatile nature
(d) None of the
above
Answer:
(a) intermolecular hydrogen bonding
Due to intermolecular
hydrogen bonding in alcohols, they exist as associated molecules.
Consequently, a large amount of energy is required to break these bonds and,
therefore, their boiling points are higher than that of the corresponding
hydrocarbons (hydrogen bonding is absent in hydrocarbons).
Question 12.
Which type of electrolyte are used in A and B ? [1]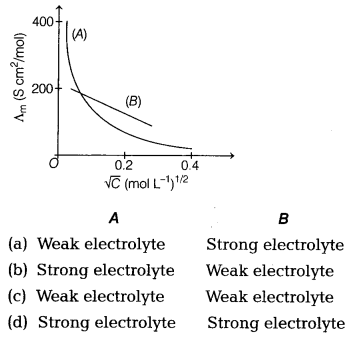
Answer:
(a) In the given graph, ‘A’ represents weak electrolyte like
CH3COOH as Λm increases steeply on dilution at low
concentration region and ‘6 ’ represents strong electrolyte like NaCl as
Λm increases slowly with dilution.
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is
followed by a corresponding Reason (R). Use the following keys to choose the
appropriate answer.
(a) Both (A) and (R) are true, (R) is the correct
explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct
explanation of (A).
(c) (A) is true, (R) is false.
(d) (A) is false, (R)
is true.
Question 13.
Assertion (A) p-nitrophenol is more acidic than phenol.
Reason (R) Nitro group helps in the stabilisation of the phenoxide ion by
dispersal of negative charge due to resonance. [1]
Answer:
(a) Both (A)
and (R) are true, (R) is the correct explanation of (A).
Question 14.
Assertion (A) In electrolysis, the quantity of electricity
needed for depositing 1 mole of silver is different from that required for 1
mole of copper.
Reason (R) The molecular weights of silver and copper are
different. [1]
Answer:
(b) Both (A) and (R) are true, (R) is not the
correct explanation of (A).
According to Faraday’s law of electrolysis the
weight of ion deposited on an electrode is directly proportional to the quantity
of electricity passed. So, 1 mole of silver = 1g equivalent of silver and 1 mole
of copper = 2g equivalent of copper.
Question 15.
Assertion (A) All naturally occurring α-amino acids except
glycine are optically active.
Reason (R) Most naturally occurring amino acids
have L-configuration. [1]
Answer:
(b) Both (A) and (R) are true, (R) is
not the correct explanation of (A).
All naturally occurring α-amino acids
except glycine are optically active. Glycine is optically inactive because
glycine does not have all four different substituents as shown below.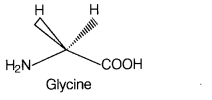
Question 16.
Assertion (A) The a-hydrogen atom in carbonyl compounds is
less acidic.
Reason (R) The anion formed after the loss of a-hydrogen atom is
resonance stabilised. [1]
Answer:
(d) (A) is false, (R) is true.
(A) is false because the a-hydrogens in carbonyl compounds are generally
acidic in nature and there will be an easy loss of these in form of
H+ ions.
The anion formed after loss of α-hydrogen atom is
resonance stabilised.
Section
B
(This section contains 7
questions with internal choice in one question. The following questions are very
short answer type and carry 2 marks each.)
Question 17.
At first order reaction is 25% complete in 40 minutes.
(a)
Calculate the value of rate constant.
(b) In what time will the reaction be
80% completed? [2]
Answer:
(a) For first order reaction,
k =
\(\frac{2.303}{t}\)log\(\frac{a}{a-x}\)
The given first order reaction
completes 25% in 40 min.
Let a = 100
a – x = 100 – 25 = 75
∴ k =
\(\frac{2.303}{40}\)log \(\frac{100}{75}\) = \(\frac{2.303}{40}\)(log 4 – log
3)
k = 0.0072 min-1
(b) For 80% completion of reaction,
a = 100
a – x = 100 – 80 = 20
k
= 0.0072 min-1
t = \(\frac{2.303}{0.0072}\)log\(\frac{100}{20}\) =
223.6 min
Question 18.
A solution containing 25.6 g of sulphur dissolved in 1000 g
of naphthalene, whose melting point is 80.1°C gave a freezing point lowering of
0.680°C. Calculate the molecular weight of sulphur, Kf for
naphthalene is 6.8 K kg mol-1. [2]
Answer:
∆Tf =
Kf ∙ m = Kf ∙ \(\frac{W_B}{M_B \cdot W_A(\text { in kJ
})}\)
0.68 = 6.8 × \(\frac{25.6}{M_B}\) × \(\frac{1000}{1000}\)
MB = \(\frac{6.8 \times 25.6}{0.68}\) = 256 g
Question 19.
(a) Name the suitable haloarene and reagent from which
2-chloroacetophenone can be prepared.
(b) Out of chlorobenzene and cyclohexyl
chloride, which one has higher dipole moment? [2]
Answer:
(a)
2-chloroacetophenone can be obtained from chlorobenzene and acetyl chloride in
the presence of anhyd. AlCl3.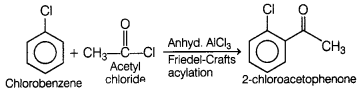
(b) In chlorobenzene, the Cl-atom is linked to a sp2-hybridised
carbon atom, whereas in cyclohexyl chloride, the Cl-atom is linked to a
sp3-hybridised
carbon atom.
As, sp2-hybridised
carbon has more s-character so, it is more electronegative, thus the density of
electrons of C—Cl bond near the Cl-atom is less in chlorobenzene than in
cyclohexyl chloride.
Hence, the C—Cl bond of cyclohexyl chloride is more
polar, i.e. it has higher dipole moment.
Question 20.
(a) Give chemical tests to distinguish between phenol and
benzoic acid. [2]
(b) Write IUPAC name of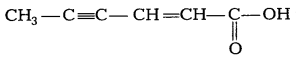
Or
Convert the following. [2]
(a) Ethyl cyanide to
ethanoic acid
(b) Butan-l-ol to butanoic acid
Answer:
(a) Phenol and
benzoic acid can be distinguished by ferric chloride test. Phenol reacts with
neutral FeCl3 to form ferric phenoxide complex giving violet
colouration.
But benzoic acid reacts with neutral FeCl3 to give a buff coloured
precipitate of ferric benzoate.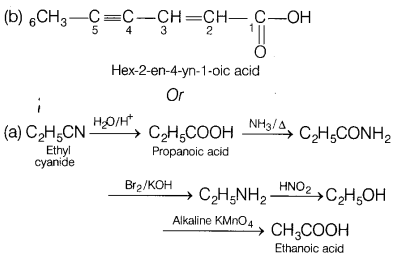
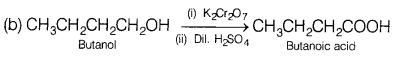
Question 21.
(a) Account for the following. [2]
Pentaacetate of glucose
does not react with hydroxyl amine.
(b) What happens when oxidation of
gluconic acid takes place?
Answer:
(a) “Pentaacetate of glucose does not
react with hydroxylamine” due to the absence of free CHO group.
(b) Glucose
and gluconic acid, both on oxidation yields a dicarboxylic acid, saccharic
acid.
Reaction involved is as follows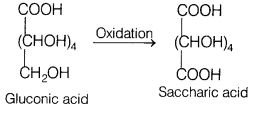
Section
C
(This section contains 7
questions with internal choice in one question. The following questions are
short answer type and carry 3 marks each.)
Question 22.
(a) What kind of isomerism exists between
[Cr(H2O)6]Cl3 (violet) and
[Cr(H2O)5Cl]Cl2 ∙ H2O
(greyish-green)? [3]
(b) Write the formula of diamminechlorido-
nitrito-N-platinum (II).
(c) Different colours observed in octahedral and
tetrahedral complexes for the same metal and same ligands. Explain.
Answer:
(a) Coordination compound
[Cr(H2O)6]Cl3 and
[Cr(H2O)5Cl]Cl2 ∙ H2O are solvate
isomers, because water is exchanged by chloride ion. This is why both of them
show different colour on exposure to sunlight.
(b) The formula is
[Pt(NH3)2Cl(NO2)].
(c) Crystal field
splitting energy in the octahedral and tetrahedral field are closely related
as
∆t = \(\left(\frac{4}{9}\right) \Delta_0\)
Higher the CFSE,
higher will be the energy radiated in d-d transition.
Hence, lower wavelength
is absorbed in octahedral complex than tetrahedral complex for the same metal
and ligands. Thus, different colours are observed.
Question 23.
(a) What happens if external potential applied becomes
greater than E°cell of electrochemical cell? [3]
(b) Write the
relation among the conductivity of a solution in the cell constant and the
resistance of solution in the cell.
(c) The resistance of a conductivity cell
containing 0.001 M KCl solution at 298 K is 1500 Ω. What is the cell constant if
the conductivity of 0.001 M KCl solution at 298 K is 0146 × 10-3 S
cm-1?
Answer:
(a) When external potential applied becomes
greater than E°cell of electrochemical cell, electrons flow from
cathode to anode, i.e. electrolytic cell.
(b) The resistance, R of a conductor varies directly with length (l) and
inversely with area of cross-section A,
i.e. R ∝ \(\frac{1}{A}\)
or R =
\(\rho \frac{1}{A}\)
where ρ = resistivity ρ = \(\frac{R A}{1}\)
(c) Given, conductivity, \(\kappa\) = 0.146 × 10-3 S
cm-1
Resistance, R = 1500Ω
∴ Cell constant, G* = K × R
=
0.146 × 10-3 × 1500
= 0.219 cm-1
Question 24.
Write the equation for the following reactions. [3]
(a)
Conversion of propan-2-ol to 2-methyl propan-2-ol
(b) Phenol is treated with
Na2Cr2O7 in the presence of sulphuric acid.
Answer:
(a) Propan-2-ol to 2-methylpropan-2-ol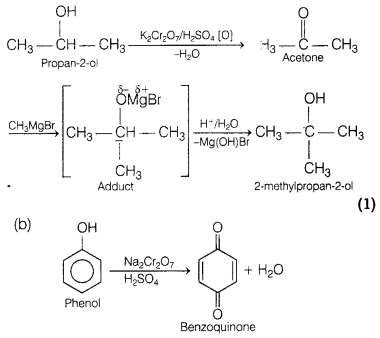
Oxidation of phenol is done with Na2Cr2O7 in
the presence of sulphuric acid and benzoquinone is formed along with water.
Question 25.
An organic compound (P) having molecular formula
C9H10O forms an orange precipitate (Q) with 2, 4-DNP
reagent. Compound (P) gives a yellow precipitate (R) when heated in the presence
of iodine and NaOH along with colourless compound (S).
Identify (P), (Q), (R)
and (S). Write down the reaction for their formation. [3]
Or
An unknown aldehyde ‘A’ on reacting with alkali gives a β-hydroxy aldehyde,
which loses water to form an unsaturated aldehyde, but-2-enal.
Another
aldehyde ‘B’ undergoes disproportionation reaction in the presence of conc.
alkali to form products C and D. C is an aryl alcohol with the formula
C7H8O. Identify A and B. Write the sequence of reactions
involved. [3]
Answer:
P = Phenyl acetone
Q = 2, 4-dinitrophenyl
hydrazone
R = Methyl iodide
S = Phenyl acetic acid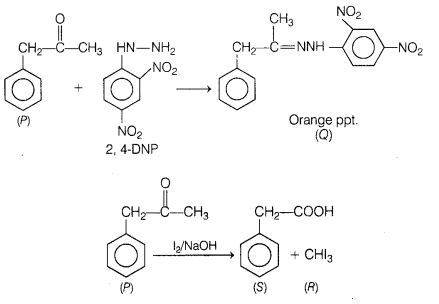
Or
A is CH3CHO (ethanal).
B is C6H5CHO
(benzaldehyde).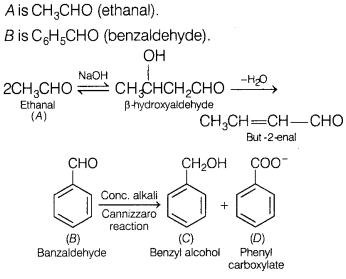
Question 26.
A simplified version of nucleic acid chain is as shown below.
[3]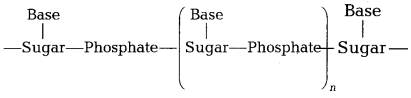
(a) Write the main structural difference between nucleoside and nucleotide.
(b) How do two nucleic acid chains bond with each other?
(c) Name the bases
present in DNA.
Answer:
(a) Nucleosides contain only sugar and a base,
whereas nucleotides contain sugar, base and a phosphate group as well.
(b)
Two nucleic acid chains are held together by hydrogen bonds between pairs of
bases.
(c) There are four nucleotides, or bases, in DNA: adenine (A),
cytosine (C), guanine (G), and thymine (T).
Question 27.
An alcohol having formula C2H6O reacts
with HBr and forms products. Write the name and structure of both products and
the mechanism of the reaction. [3]
Answer:
The involved chemical reaction
is![]()
The mechanism of the given reaction is as follows
step 1
HBr → H+ + Br–
Step 2 Protonation of alcohol![]()
Step 3 Nucleophilic attack of Br–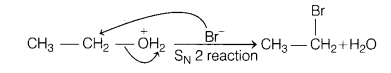
Question 28.
Prove that time required to complete 3/4 of a first order
reaction is twice its half-life period. [3]
Answer:
For first order
reaction, rate constant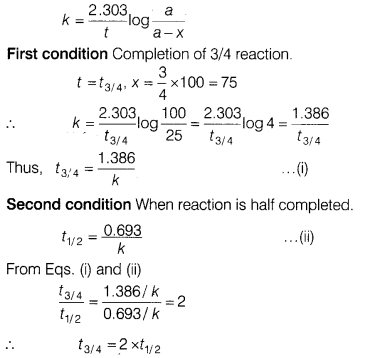
Thus, time needed to compare 3/4 of reaction is twice its half-life period.
Section
D
(The following questions are
case-based questions. Each question has an internal choice and carries 4(1+1+2)
marks each. Read the passage carefully and answer the questions that
follow.)
Question 29.
Crystal field theory is a theory that describes the breaking
of the degeneracy of electronic orbitals that occurs in transition metal
coordination complexes, most often as a consequence of the presence of
ligands.
Crystal field splitting energy (CFSE) is the parameter that is used
to correlate a variety of spectroscopic, thermodynamic, and magnetic properties
of complexes. The essential feature of crystal field theory is that there is a
competition between the magnitude of the CFSE and the pairing energy, which is
the energy required to accommodate two electrons in one orbital.
The energy
splitting is shown schematically in the figure given below.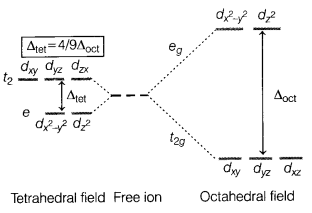
Answer the following questions. [4]
(a) On the basis of this theory, write the electronic configuration of d4 system in terms of t2g and eg if ∆0 < P.
Or
Write the electronic configuration of the central atom
K4[Fe(CN)6] w.r.t crystal field theory. [4]
(b) What is
the crystal field stabilisation energy for high spin d4 octahedral
complex?
(c) How does the magnitude of the splitting decide the actual
configuration of d-orbitals in an octahedral field for a coordination
entity?
Answer:
(a) If ∆0 < P, the fourth electron enters
one of the eg orbitals giving the configuration \(t_{2 g}^3 e_g^1\).
Or
The electronic configuration of the central atom is K4[Fe(CN]6 on the basis of crystal field theory is \(t_{2 g}^6 e_g^0\)
(b) CFSE = 3(-0.4)∆0 + 0.6∆0
= -1.2∆o +
0.6∆o = -0.6∆0
(c) The magnitude of splitting decides the actual configuration of d-orbital
in an octahedral field for a coordination entity on the following basis.
If
∆o < P (P is the energy required for pairing of electrons), then
fourth electron enters one of the eg orbitals giving the
configuration \(t_{2 g}^3 e_g^1\). It means first four orbitals are singly
occupied and no pairing will take place, forming high spin complexes. Such
ligands for which ∆0 < P are called weak field ligands.
If ∆0 > P, the 4th electron pairs up in one of the
t2g orbitals giving the configuration \(t_{2 g}^4 e_g^0\) thereby
forming low spin complexes. Such ligands for which
∆0 > P are
called strong field ligands.
Question 30.
Electrochemical cell potentials have no simple relationship
with temperature but depend on the interplay between the sign and magnitude of
the isothermal temperature coefficient, dE°/dT, and on the magnitude of the
reaction quotient, Q. An electrochemical cell is said to act reversibly if the
net cell reaction is reversed when the current through the cell is made to flow
in the opposite direction.
When no current is being drawn, such a cell is in a true equilibrium state. When no current is being drawn from a reversible cell, the potential difference across its terminal, or open-circuit potential, is known as the electromotive force or emf of the cell.
The emf of any particular reversible cell is a quantitative measure of the tendency of the cell reaction to occur and may be related to the free energy change for this process.
The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consists of a copper pot filled with a copper (II) sulphate solution, in which is immersed an unglassed earthenware container filled with sulphuric acid and a zinc electrode.
Several scientists performed experiments that used a Daniell galvanic cell working in different electrolyte concentrations for comparing results with the theoretical values calculated by the Nernst equation. The cell potential decreases drastically when the Cu2+ concentration was reduced and the temperature was above 80°C.
In addition, temperature indirectly has an influence through the activity coefficients, ionic strength and dilution of the solution.
Answer the following questions. [4]
(a) An electrochemical cell generally consists of a cathode and an anode.
Where is the reduction takes place?
(b) What is the effect of increase in
concentration of zinc ions on the electrode potential of zinc electrode for
which \(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\circ}\) equals -0.76 V ?
(c) How
does the reduction potential increase w.r.t. Nernst equation?
Or
Imagine you are a member of a project based on electrochemical cell and you
are asked to construct an electrochemical cell. What will be its main four
components? [4]
Answer:
(a) Cathodes are usually metal electrodes. It is
the electrode where reduction takes place.
(b) According to Nernst equation,
the electrode potenial of zinc depends on the concentration of zinc
ions.![]()
Hence, electrode potential will increase with increase in
concentration of Zn2+ ions.
(c) For the electrode
reaction,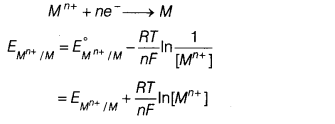
∴ Electrode potential can be increased either by increasing metal ion
concentration, [Mn+] or temperature T.
Or
The four components of an electrochemical cell are as follows
(a) Anode An
electrode where oxidation occurs.
(b) Cathode An electrode where reduction
occurs.
(c) Electrolyte An electrolyte is a charged mobile ion that functions
as a conducting medium.
When dissolved in polar solvents such as water, an
electrolyte produces ions, resulting in an electrically conducting solution.
(d) Salt-bridge A salt-bridge connects the oxidation and reduction halves of an
electrochemical cell, completing the electrochemical circuit. It contains a
saturated salt solution, such as KCl.
Section
E
(The following questions are
long answer type and carry 5 marks each. All questions have an internal
choice.)
Question 31.
Attempt any five of the following. [5]
(a) Why
Mn2+ is more stable than Fe2+ towards +3 oxidation
state?
(b) Why the enthalpy of atomisation is lowest for Zn in 3d-series of
the transition elements?
(c) Identify the metal in MO3F and
justify your answer.
(d) Explain why transition metals form a large number of
complexes.
(e) Why the E° value for the Mn3+ /Mn2+
couple is much more positive than that for Cr3+ /Cr2+
couple?
(f) Complete the following.
3Mn\(\mathrm{O}_4^{2-}\) +
4H+ →
(g) Which of the following cations are coloured in aqueous
solution and why?
Sc3+, V3+, Ti4+,
Mn2+
Answer:
(a) Electronic configuration of Mn2+ =
[Ar]183d5
Electronic configuration of Fe2+ =
[Ar]183d6
Mn2+ having half-filled d-orbitals
will be more stable than Fe2+, as it has half-filled d-orbitals.
(b) Zinc has completely filled d-orbitals, which limits its tendency to form
metallic bonds. Thus, it requires least enthalpy to get atomised.
(c)
MO3F is MnO3F. In MO3F,
Let the oxidation
state of M is x.
x + 3 × (-2) + (-1) = 0
or, x = + 7, i.e. M is in
oxidation state of +7. Hence, the given compound is MnO3F.
(d)
Transition elements form a large number of complexes due to the comparatively
smaller sizes of the metal ions, their high ionic charges and the availability
of d-orbitals for bond formation.
(e) The value of \(E_{\mathrm{Cr}^{3+} /
\mathrm{Cr}^{2+}}^{\circ}\) is negative (-0.4 V).
It shows the stability of
\(E_{\mathrm{Cr}^{3+}}^0\) ions in solution cannot be reducing agent of
Mn3+ has high positive value due to extra stability of half-filled
electronic configuration. Thus, Cr3+ is the most stable,
Mn3+ is least stable.
(f) 3Mn\(\mathrm{O}_4^{2-}\) +
4H+ → 2Mn\(\mathrm{O}_4^{-}\) + MnO2 + 2H2O
(g) Electronic configurations of the ions are :
Sc3+ =
[Ar]3d04s0, V3+ =
[Ar]3d24s0,
Ti4+ =
[Ar]3d04s0, Mn2+ =
[Ar]3d54s0
Only V3+ and Mn2+ can
undergo d-d transition, hence they are coloured, while Ti4+ and
Sc3+ do not have any electron, thus they are colourless.
Question 32.
(a) Gas (A) is more soluble in water than gas (B) at the same
temperature. Which one of the two gases will have the higher value of
KH (Henry’s constant) and why?
(b) In non-ideal solution, what
type of deviation shows the formation of maximum boiling azeotropes?
(c) What type of azeotropic mixture will be formed by a solution of acetone and
chloroform? Justify on the basis of strength of intermolecular interactions that
develop in the solution. [5]
Or
(a) How is the vapour pressure of a solvent affected when a non-volatile
solute is dissolved in it?
(b) When 2.56 g of sulphur was dissolved in 100 g
of CS2, the freezing point gets lowered by 0.383 K. Calculate the
formula of sulphur (Sx).
(Kf for CS2 = 3.83
K kg mol-1,
Atomic mass of sulphur =32 g mol-1) [5]
Answer:
Solubility is inversely proportional to KH, i.e. Henry’s
constant of the gas.
(a) Greater the value of KH, lower is the
solubility of the gas. As gas (A) is more soluble in water than gas (B) at the
same temperature, hence the gas (A) has lower value of KH. In other
words, gas (B) has higher value of KH than gas (A) at the same
temperature.
(b) In non-ideal solutions, the solutions that show large
negative deviation from Raoult’s law form maximum boiling azeotropes, e.g.
Mixture of nitric acid and water.
(c) The mixture of acetone and chloroform
is an example of maximum boiling azeotrope. It shows negative deviation from
Raoult’s law because of increase in intermolecular forces of attraction between
acetone and chloroform, since they form hydrogen bonds between them.
Or
(a) When a non-volatile solute is added to a solvent, its vapour pressure
decreases because some of the surface sites are occupied by solute molecules.
Thereby, the fraction of the surface covered by the solvent molecules gets
reduced.
Thus, less space is available for the solvent molecules to vaporise.
Therefore, vapour pressure is also reduced.
(b) Given, Weight of solvent (W1) = 100g
Weight of solute
(w2) = 2.56 g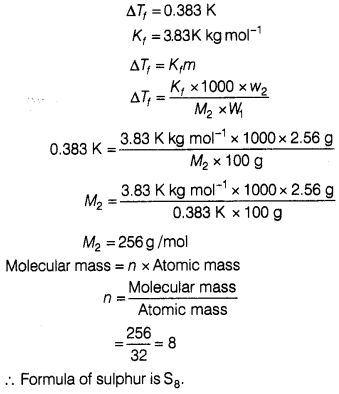
Question 33.
(a) Give reasons for the following observations.
(i)
Aniline is a weaker base than cyclohexyl amine.
(ii) It is difficult to
prepare pure amines by ammonolysis of alkyl halides.
(iii) Ethylamine is
soluble in water, whereas aniline is not soluble in water.
(b) Arrange the
following in
(i) increasing order of their solubility in water.
C6H5NH2,
C2H5NH2,
(C2H5)2NH
(ii) increasing order of basic
strength. Aniline, p-nitroaniline and p-toluidine [5]
Or
An organic compound A’ with molecular formula C6H7N is
treated with NaNO2 and dil. HCl and compound ‘B’ is formed. The
compound ‘B’ further reacts with H3PO2 and water, an
aromatic compound ‘C’ is formed. On Friedel-Crafts alkylation, the compound ‘C’
gets converted into compound ‘D’ having molecular formula
C7H8. In the presence of sunlight, this compound gets
converted into another compound Write the structure of compounds A to E. Write
the chemical equations involved. [5]
Answer:
(a) (i) In aniline, the lone
pair present on nitrogen is involved in delocalisation. Thus, electrons are not
available for donation. There is no such delocalisation in cyclohexylamine.
Hence, aniline is weaker base than cyclohexylamine.
(ii) It is difficult to
prepare pure amines by ammonolysis of alkyl halides because it is a nucleophilic
substitution reaction and ammonia behaves as a nucleophile to form 1° amine.
Now, this T amine behaves as a \(\mathrm{Nu}^{\ominus}\) and reacts with alkyl
halide to give 2° amine, which on further reaction yields 3° amine and
quaternary ammonium salt.
(iii) Solubility of compound in water, depends on
H-bonding. Bulkiness of the group attached to such bond decreases the extent of
H-bonding.
Aniline is not soluble in water, while ethylamine is soluble. This
is because ethylamine forms intermolecular hydrogen bonds with water, while in
aniline, the aryl group possesses steric hindrance and also high molar mass and
hence, does not resuit in the formation of H-bonding. (1)
(b) (i) C6H5NH2 <
(C2H5)2NH <
C2H5NH2
Aromatic amines are insoluble in
water because of bulky hydrocarbon part (hydrophobic) which retards the
formation of H-bonding. However, increase in size of hydrophobic alkyl part in
aliphatic amines decreases solubility in water.
(ii) p-nitroaniline <
aniline < p-toluidine.
The presence of electron-donating —CH3
group increases the electron density on N-atom in p-toluidine. Hence,
p-toluidine is more basic than aniline. In p-nitroaniline, —NO2 group
decreases the electron density over the N-atom. Hence, p-nitroaniline is less
basic than aniline.
Or
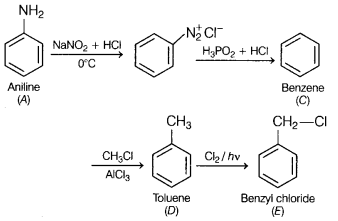
A = Aniline;
B = Benzene diazonium chloride;
C = Benzene;
D = Toluene
and
E = Benzyl chloride.