CBSE Sample Papers for Class 12 Chemistry Set-12
Class 12thCBSE Sample Papers for Class 12 Chemistry Set-12
CBSE Sample Papers for Class 12 Chemistry Set 12 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section
A
(The following questions are
multiple-choice questions with one correct answer. Each question carries 1 mark.
There is no internal choice in this section.)
Question 1.
Major product formed on monochlorination of toluene in
sunlight followed by hydrolysis in aqueous NaOH is [1]
(a) o-cresol
(b)
m-cresol
(c) 2, 4-dihydroxytoluene
(d) benzyl alcohol
Answer:
(d)
benzyl alcohol
Monochlorination of toluene in sunlight gives benzyl chloride. On hydrolysis
with aq. NaOH, benzyl chloride shows nucleophilic substitution reaction to give
benzyl alcohol.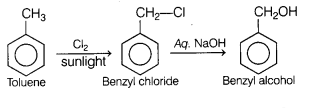
Question 2.
Arrange the following in decreasing order of basic strength in
aqueous medium [1]
A. (CH3)2NH
B.
CH3NH2
C.
C6H5NHCH3
D.
NC—CH2NH2
Choose the correct option.
(a) A > B
> C > D
(b) D > C > B > A
(c) C > A > B > D
(d)
B > D > A > C
Answer:
(a) A > B > C > D
2° amine is more basic than 1° amine, i.e. (CH3)2NH is
more basic than CH3NH2. Due to -1 effect of CN group,
NC—CH2NH2 is less basic than CH3NH2.
Further C6H5NHCF3 is less basic than both
CH3NH2 and (CH3)2NH due to
delocalisation of Ione pair of electrons present on the nitrogen atom into
benzene ring. Hence, the decreasing order of amines is
(CH3)2NH > CH3NH2 >
C6H5NHCH3 >
NC—CH2NH2
2° amine is more basic than 1° amine, i.e. (CH3)2NH is
more basic than CH3NH2. Due to -1 effect of CN group,
NC—CH2NH2 is less basic than CH3NH2.
Further C6H5NHCF3 is less basic than both
CH3NH2 and (CH3)2NH due to
delocalisation of Ione pair of electrons present on the nitrogen atom into
benzene ring. Hence, the decreasing order of amines is
(CH3)2NH > CH3NH2 >
C6H5NHCH3 >
NC—CH2NH2
Question 3.
Which of the following statements concerning methylamine is
correct? [1]
(a) Methylamine is stronger base than NH3.
(b)
Methylamine is less basic than NH3.
(c) Methylamine is slightly
acidic.
(d) Methylamine forms salts with alkali.
Answer:
(a)
Methylamine is stronger base than NH3.
Methylamine is stronger base than ammonia due to presence of alkyl group which has electron releasing (+ 1 effect). As a result, the electron density increases on the nitrogen atom and therefore, they can donate electron pair more easily than ammonia.
Question 4.
In the graph showing Maxwell-Boltzmann distribution of energy.
[1]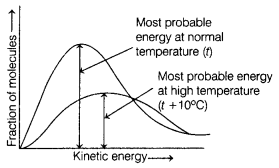
(a) area under the curve remains same with increase in
temperature
(b) area under the curve increases with increase in
temperature
(c) area under the curve decreases with , increase in
temperature
(d) with decrease in temperature, curve broadens and shifts to
the right hand side
Answer:
(a) area under the curve remains same with
increase in temperature
Area under the curve remains same with increase in temperature but the curve broadens and shifts to right-hand side.
Question 5.
The major product formed when acetone is reduced in presence
of NaBH4 is [1]
(a) 1-propanol
(b) 2-propanol
(c)
propionaldehyde
(d) propanoic acid
Answer:
(b) 2-propanol
Acetone undergoes reduction in presence of strong reducing agents like
NaBH4 or LiAlH4 to give 2-propanol. The reaction
is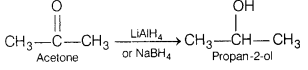
Question 6.
Cd is not regarded as transition element due to [1]
(a)
completely filled d-orbital in the excited state.
(b) completely filled
orbital in ground state as well as in its common oxidation state.
(c)
completely filled s-orbital.
(d) completely filled 5d-orbital.
Answer:
(b) completely filled orbital in ground state as well as in its common oxidation
state.
Cd, Zn and Hg are not regarded as transition elements due to completely filled orbital in ground state as well as in its common oxidation state.
Question 7.
Match the following columns. [1]
| A. Ethyl Alcohol | 1. Ethanoic acid |
| B. Acetic acid | 2. Ethanol |
| C. Formic acid | 3. Methanoic acid |
| D. Butyl aldehyde | Butanal |
Codes
(a) A – 2, B – 1, C – 3, D – 4
(b) A – 1, B – 2, C – 3, D – 4
(c) A – 4, B – 3, C – 2, D – 1
(d) A – 2, B – 4, C – 3, D – 1
Answer:
(a) A – 2, B – 1, C – 3, D – 4
The correct match is A-2, B-1, C-3, D-4.
Question 8.
Electrode potential for Mg electrode varies according to the
equation: [1]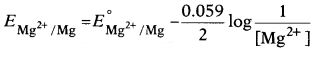
The correct graphical representation of \(E_{\mathrm{Mg}^{2+}
/ \mathrm{Mg}}\) versus log [Mg2+] will be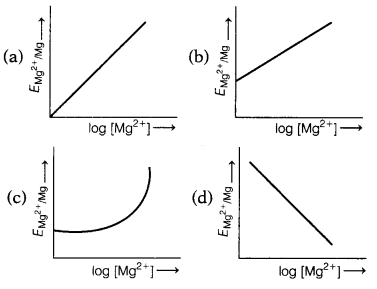
Answer:
(b)
\(E_{\mathrm{Mg}^{2+} / \mathrm{Mg}}\) = \(E_{\mathrm{Mg}^{2+} / \mathrm{Mg}}^0\) + \(\frac{0.059}{2}\)log[Mg2+]
On comparing the equation with equation of the straight line y = mx + c we get, the graph of \(E_{\mathrm{Mg}^{2+} / \mathrm{Mg}}\) versus log [Mg2+] is a straight line with a positive slope and intercept \(E_{\mathrm{Mg}^{2+}}^0 / \mathrm{Mg}\).
Question 9.
Which one of the following compound is more reactive towards
SN2 reaction? [1]
(a)
CH3CH2CH2CH2Br
(b)
CH3CH2CH(Br)CH3
(c)
(CH3)3CBr
(d)
(CH3)2CHCH2Br
Answer:
(a)
CH3CH2CH2CH2Br
Primary halides are most reactive towards \(\mathrm{S}_{\mathrm{N}} 2\)
reaction as they are sterically less hindered as compared to secondary and
tertiary halides.
Therefore,
CH3CH2CH2CH2Br will be most reactive
towards \(S_N 2\) reaction.
Question 10.
The number of chloride ion produced by complex
tetraamminedichloroplatinum (IV) chloride in an aqueous solution is [1]
(a)
two
(b) four
(c) one
(d) three
Answer:
(a) two
Formula of tetramminedichloroplatinum (IV) chloride is
[Pt(NH3)4Cl2]Cl2.
In aqueous
solution, the above compound ionises as
[Pt(NH3)4Cl2]Cl2
\(\rightleftharpoons\)
[Pt(NH3)4Cl2]2+ +
2Cl–
So, the number of chloride ion is 2.
Question 11.
Rate law for reaction A + 2B → C is found to be Rate = k[A]
[B]
If concentration of the reactant ‘B’ is doubled on keeping the
concentration of ‘A’ constant, then the value of rate constant will be [1]
(a) the same
(b) doubled
(c) quadrupled
(d) halved
Answer:
(a)
the same
Rate constant of a reaction does not depend upon concentrations of the reactants.
Question 12.
When 1 mole CrCl3 ∙ 6H2O is treated
with excess of AgNO3, 3 moles of AgCl are obtained. The formula
of the complex is [1]
(a) [CrCl3(H2O)3] ∙
3H2O
(b)
[CrCl2(H2O)4]Cl2 ∙
H2O
(c) [CrCl (H2O)5]Cl2 ∙
H2O
(d) [Cr (H2O)6]Cl3
Answer:
(d) [Cr (H2O)6]Cl3
3 moles of AgCl means 3 Cl are ionised in the solution hence, the formula of the complex will be [Cr(H2O)6]Cl3.
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is
followed by a corresponding Reason (R) use the following keys to choose the
appropriate answer.
(a) Both (A) and (R) are true, (R) is the correct
explanation of (A).
(b) Both (A) and (R) are true, (R) is not the correct
explanation of (A).
(c) (A) is true, (R) is false.
(d) (A) is false, (R)
is true.
Question 13.
Assertion (A) Actinoids form relatively less stable complexes
as compared to lanthanoids.
Reason (R) Actinoids can utilise their
5f-orbitals along with 6d-orbitals in bonding but lanthanoids do not use their
4f-orbital for bonding. [1]
Answer:
(d) (A) is false, (R) is true.
(A) is false, (R) is true as actinoids possess greater tendency to form complexes as compared to lanthanoids because actinoid ions possess high charge and small size.
Question 14.
Assertion (A) p-nitrophenol is more acidic than phenol.
Reason (R) Nitro group helps in the stabilisation of the phenoxide ion by
dispersal of negative charge due to resonance. [1]
Answer:
(a) Both (A)
and (R) are true, (R) is the correct explanation of (A).
Both (A) and (R) are true and (R) is the correct explanation of (A).
Question 15.
Assertion (A) Acylation of amines gives a monosubstituted
product, whereas alkylation of amines gives polysubstituted product.
Reason
(R) Acylation occurs at N-atom, whereas alkylation occurs at o/p position.
[1]
Answer:
(c) (A) is true, (R) is false.
(A) is true but (R) is false as both occurs at N-atom. Acylation is a monosubstituted product because N is in conjugation with acyl group due to which nucleophilicity of N-atom is reduced.
Question 16.
Assertion (A) All naturally occurring α-amino acids except
glycine are optically active.
Reason (R) Most naturally occurring amino acids
have L-configuration. [1]
Answer:
(b) Both (A) and (R) are true, (R) is
not the correct explanation of (A).
Both (A) and (R) are true, (R) is not the correct explanation of (A).
All naturally occurring a-amino acids are optically active since the a-C atom is asymmetric. These exist both in ‘D’ and ‘L’ forms.
Section
B
(This section contains 5
questions with internal choice in one question. The following questions are very
short answer type and carry 2 marks each.)
Question 17.
Give reason for the following.
(a) p-dibromobenzene has
higher melting point than o-isomer. [2]
Answer:
Due to more symmetry in
p-isomer, it fits better in crystal lattice than its o-isomer. Hence, have
higher melting point.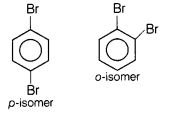
(b) Haloarenes are less reactive than haloalkanes and haloalkenes.
Answer:
In haloarenes, the electron pairs on halogen atom are in conjugation
with π-electrons of the ring as shown in figure.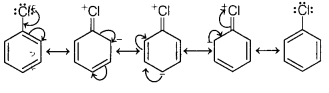
C—Cl bond acquires a partial double bond character due to
resonance. As a result, the bond cleavage in haloarenes is difficult than
haloalkanes and haloalkenes therefore they are less reactive towards
nucleophilic substitution reaction.
Question 18.
Explain how and why the rate of reaction for a given reaction
gets affected when
(a) temperature is raised.
(b) reaction progresses.
[2]
Answer:
(a)
The rate of reaction for any reaction increases with
rise in temperature. At higher temperatures, larger fraction of colliding
particles can cross the energy barrier (i.e. the activation energy), which leads
to faster rate.
(b) The rate of reaction decreases as the reaction progresses. The rate of a reaction depends on the concentration of reactants. As the reaction progresses, the concentration of reactants decreases because the reactants start getting converted to products. Hence, the rate decreases.
Question 19.
(a) Write the cell reaction of a lead storage battery when it
is discharged.
(b) How does the density of the electrolyte change when the
battery is discharged? [2]
Answer:
(a)![]()
(b) Density of electrolyte decreases because water is formed
and sulphuric acid is consumed during discharge of the battery.
Question 20.
Account for the following.
(a) Sucrose is dextrorotatory
but the mixture obtained after hydrolysis is laevorotatory.
(b) Amino acids
behave like salts rather than simple amines or carboxylic acids in aqueous
solution. [2]
Answer:
(a) Sucrose is dextrorotatory having [α]D
= + 665°. On hydrolysis with dilute acids or enzymes, it gives equimolar mixture
of D-(+)-glucose, and D-(-)-fructose. Since, D-(-) fructose has larger specific
rotation than D-(+)-glucose, the resulting mixture has specific rotation of –
39.9°. Therefore, the mixture is laevorotatory.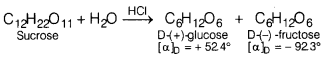
(b) In aqueous solution, the —COOH group of an amino acid loses a proton and
-NH2 group accepts a proton to form Zwitter ion (salt).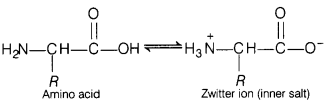
Or
When sucrose is hydrolysed the optical rotation values are measured using a polarimeter and are given in the following table. [2]
| Time (Hours) | Specific rotation |
| 0 | +66.5° |
| ∞ | -39.9° |
(a) Account for the two specific rotation values.
(b) What is the specific
name given to sucrose based on the above observation?
Answer:
(a) Sucrose
is dextrorotatory but after hydrolysis gives dextrorotatory glucose and
laevorotatory fructose. Since, the laevorotation of fructose (-92.4°) is more
than dextrorotation of glucose (+ 52.5°), the mixture is laevorotatory.
(b)
The hydrolysis of sucrose brings about a change in the sign of rotation from
dextro (+) to laevo (-). Thus, the product is named as invert sugar.
Question 21.
The hexaaquamanganese(II) ion contains five unpaired
electrons, while the hexacyano manganese (II) ion contains only one unpaired
electron. Explain using crystal field theory. [2]
Answer:
Mn (II) ion has
3d5 configuration. In the presence of H2O molecules
(acting as weak field ligands), the distribution of these five electrons is
\(t_{2 g}^3 e_g^2\) the electrons remain unpaired to form a high spin
complex.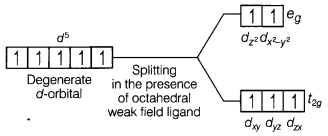
However, in the presence of CN– (acting as strong
field ligands), the distribution of these electrons is \(t_{2 g}^5 e_g^0\), i.e.
two t2g orbitals contains paired electrons while the third
t2g orbital contains one unpaired electron. The complex formed is a
low spin complex.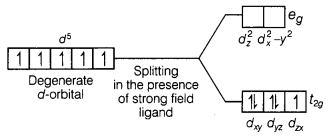
Section
C
(This section contains 7
questions with internal choice in one question. The following questions are
short answer type and carry 3 marks each.)
Question 22.
Give reasons of the following observations.
(a) Alkylamine
is more basic than ammonia.
(b) Aniline is a weaker base than cyclohexyl
amine.
(c) Electrophilic substitution in case of aromatic amines takes place
more readily than benzene. [3]
Answer:
(a)
Due to electron releasing
inductive effect (+ l) of alkyl group, the electron density on the nitrogen atom
increases and thus, it can donate the Ione pair of electrons mote easily than
ammonia. Hence, alkylamine is more basic than ammonia
(b) In aniline, the lone pair of electrons on the N-atom is delocalised over the benzene ring. As a result, the electron density on the nitrogen decreases. But in cyclohexylamine, the lone pair of electrons on N-atom is readily available due to absence of resonance. Hence, aniline is weaker base than cyclohexylamine.
(c) Aniline exists as a resonance hybrid of the following five
structures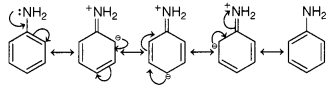
The electron density is maximum at ortho and para-positions
to the —NH2 group.
But in benzene there is no delocalisation of
electron at any position and hence electrophilic substitution in case of
aromatic amines takes place more readily than benzene.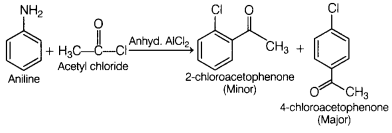
Question 23.
Answer the following questions. [3]
(a) Thermodynamic
feasibility of the reaction alone cannot decide the rate of the reaction.
Explain with the help of one example. (1)
Answer:
Thermodynamic
feasibility of a reaction depends on Gibbs free energy, i.e. AG must be negative
for spontaneous process. Kinetic feasibility depends on the activation energy of
reaction, the lesser is the activation energy, the greater is the feasibility of
reaction, i.e. Diamond → Graphite, ∆G = -ve
This process is thermodynamically
feasible but it is very slow due to its high activation energy.
(b) A first order reaction is found to have a rate constant, k = 5.5 ×
10-14 s-1. Find the half-life of the reaction. (1)
Answer:
Given, k = 5.5 × 10-14 s-1
For first order
reaction,
t1/2 = \(\frac{0.693}{k}\) = \(\frac{0.693}{5.5 \times
10^{-14}}\)
= 0.126 × 1014s
= 1.26 × 1013s
(c) If half-life period of a first order reaction is x and \(\frac{3}{4}\)th
life period of the same reaction is y, how x and y related to each other?
(1)
Answer:
For first order reaction, k = \(\frac{0.693}{t_{1 / 2}}\) =
\(\frac{0.693}{x}\)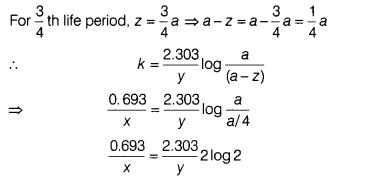
\(\frac{0.693}{x}\) = \(\frac{0.693 \times 2}{y}\)
⇒ y =
2x
Question 24.
Using valence bond theory, explain with respect to
[CO(NH3)6]3+, type of hybridisation, magnetic
moment value, and type of complex (i.e. inner or outer orbital complex). (3)
Answer:
Molecular orbital electronic configuration of Co3+ in
[Co(NH3)6]3+ is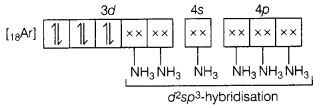
Number of unpaired electron = 0
Magnetic property =
Diamagnetic
Type of hybridisation: sp3d2
Magnetic
moment value: O as number of unpaired electrons is nil.
Type of complex:
inner orbital complex.
Question 25.
(a) Identify the major product when chlorobenzene undergoes
Friedel-Crafts acylation reaction. (3)
Write the reagents which are used to
carry out the reaction.
Answer: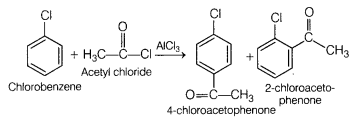
Reagents required to carry out the reaction are acetyl
chloride in presence of anhydrous aluminium chloride.
(b) Why is C—X bond in haloarenes extremely less reactive towards
nucleophilic substitution reactions?
Answer:
In C—X bond, C-atom attached
to halogen is sp2-hybriciised. The sp2-hybridised carbon
with a greater s-character is more electronegative and can hold the electron
pair of C—X bond more tightly than sp3-hybridised carbon in
haloalkane with less s-character.
Hence, it is extremely less reactive
towards nucleophilic substitution reactions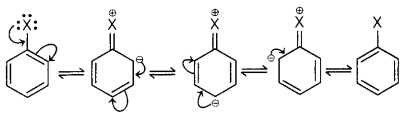
Also, in Haloarenes, C—X bond is having a π-bond character
due to delocalized electron pair of X X(halo) atom. Due to this breaking C—X
bond gets together.
Or
(a) Name the possible substrate which will give 2-methylpropene on reaction
with KOH. Write the reaction involved. (3)
Answer: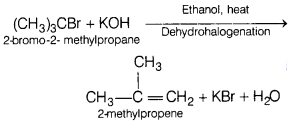
(b) Grignard reagents should be prepared under anhydrous conditions. Why?
Answer:
Grignard reagents are very reactive. They react with moisture present
in the apparatus to form alkanes![]()
Thus, Grignarci reagents must be prepared under anhydrous
conditions.
Question 26.
The partial pressure of ethane over a saturated solution
containing 6.56 × 10-3g of ethane is 1 bar.
If the solution were
to contain 5.0 × 10-2 g of ethane, then what will be the partial
pressure of the gas? (3)
Answer:
According to Henry’s law, for the same
solutions having different concentrations of solute.
m ∝ p ⇒ m = Kp
6.56 ×
10-3g = K × 1 bar ⇒ K = 6.56 × 10-3g bar-1
Again, when m’= 5.00 × 10-2g, p’ = ?
m’ = K × p’
5.00 ×
10-2 g = 6.56 × 10-3 g bar-1 × p’
p’ =
\(=\frac{5.00 \times 10^{-2}}{6.56 \times 10^{-3}}\) = 7.62 bar
Question 27.
Write the structures of A, B and C in the following
reactions. (3)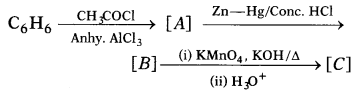
Answer: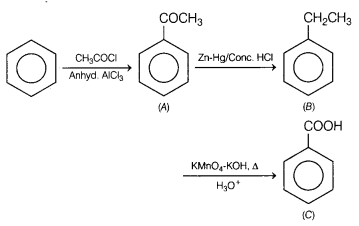
Question 28.
How are the following conversions carried out?
(a) Benzyl
chloride to benzyl alcohol
(b) Ethyl magnesium chloride to propan-1 -ol
(c) Propene to propan-2-ol (3)
Answer:
(a)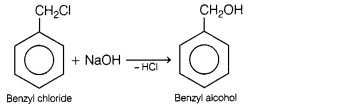
(b)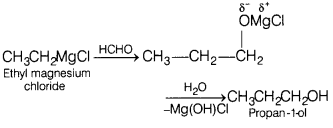
(c)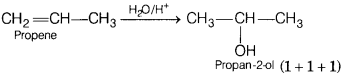
Section
D
(The following questions are
case-based questions. Each question has an internal choice and carries 4(1+1+2)
marks each. Read the passage carefully and answer the questions that
follow.)
Question 29.
Proteins are high molecular mass complex biomolecules of
amino acids. The important proteins required for our body are enzymes, hormones,
antibodies, transport proteins, structural proteins, contractile proteins etc.
Except for glycine, all α-amino acids have chiral carbon atom and most of them
have L-configuration.
The amino acid exists as dipolar ion called Zwitter ion, in which a proton goes from the carboxyl group to the amino group. A large number of α-amino acids are joined by peptide bonds forming polypeptides. The peptides having very large molecular mass (more than 10,000) are called proteins. The structure of proteins is described as primary structure giving sequence of linking of amino acids; secondary structure giving manner in which polypeptide chains are arranged and folded; tertiary structure giving folding, coiling or bonding polypeptide chains producing three dimensional structures and quaternary structure giving arrangement of sub-units in an aggregate protein molecule.
When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix gets uncoiled and protein loses its biological activity. This is called denaturation of protein. The denaturation causes change in secondary and tertiary structures but primary structures remains intact.
Examples of denaturation of protein are coagulation of egg white on boiling, curdling of milk and formation of cheese when an acid is added to milk. (4)
Answer the following questions.
(a) In which form do amino acids exist?
How is the charge distributed in amino acids?
Answer:
The amino acids
exists as dipolar ion called Zwitter ion, in which a proton goes from the
carboxyl group to the amino group.
(b) What information is furnished by secondary structure of proteins?
Answer:
Secondary structure of proteins tells us about the manner in which
the polypeptide chains are arranged and folded, i.e. If it is helical or pleated
structure.
(c) What happens when a protein is subjected to harsh changes? Write the name
of the process involved.
Answer:
When a protein is subjected to physical
or chemical changes, the hydrogen bonds present in them gets disturbed. As a
result of this the globules gets unfolded and helix gets uncoiled, leading to
loss in activity of protein. This process is called as denaturation of protein.
e.g. Formation of cheese.
Or
How is quaternary structure of proteins formed?
Answer:
Some of the
proteins are composed of two or more polypeptide chains referred to as subunits.
The spatial arrangement of these subunits with respect to each other is known as
quartemary structures. These are 3-D structures. These subunits are held
together by H-bonding and van der Waal’s forces.
Question 30.
Raj is investigating the melting point of different salt
solutions. He makes a salt using 20 mL of water with a known mass of KCl salt.
He puts the salt solution into a freezer and leaves it to freeze.
He takes the frozen salt solution out of the freezer and measures the
temperature when the frozen salt solution melts. He repeats each experiment and
gets following observations.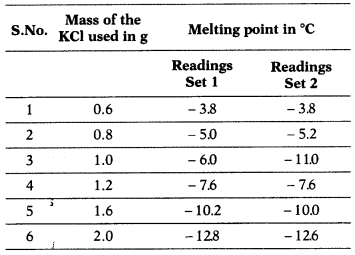
Assuming the melting point of pure water as 0°C, answer the
following questions.
(a) One temperature in the second set of results does
not fit the pattern.
Which temperature is that? Justify your answer. (4)
Answer:
Temperature -11.0°C does not fit the pattern. There has to be more
depression in freezing point and hence a sudden drop in temperature is observed.
The melting point also decreases on addition of salt. But trend observed on
addition of 1.0 g of salt is different than that observed at other amounts.
Or
Why did Raj collect two sets of results?
(b) What is the predicted melting
point if 2.4 g of salt is added to 20 mL of water? Justify your answer.
(c)
In place of KCl, if Raj had used fructose, what would have been the melting
point of the solution with 0.6 g fructose in it? (4)
Answer:
Taking
readings in two sets helps to avoid error in data collection and gives more
accuracy in results.
(b) Depression in freezing point is directly proportional to molality.
0.6
g depression is 3.8°C.
1.2 g depression is 7.6°C.
2.4 g depression will be
7.6 × 2 = 15.2° C.
(c)
Section
E
(The following questions are
long answer type and carry 5 marks each. All questions have an internal
choice.)
Question 31.
An alkene ‘A’ (Mol. formula C5H10) on
ozonolysis gives a mixture of two compounds, ‘B’ and ‘C. Compound ‘B’ gives
positive Fehling test and forms iodoform on treatment with I2 and
NaOH. Compound ‘C’ does not give Fehling test but forms iodoform. Identify the
compounds A, B and C and write all the chemical equations involved. (5)
Answer:
Compound B gives Fehling test, which means it is aldehyde. Also, it
forms an iodoform, so compound B is acetaldehyde.
Compound C does not give
Fehling test but gives iodoform, so it must be a ketone in which a methyl group
is attached to carbonyl carbon.
A = CH3CH =
C(CH3)2
B = CH3CHO, C =
CH3COCH3
Reactions for ozonolysis and formation of
iodoform from B and C are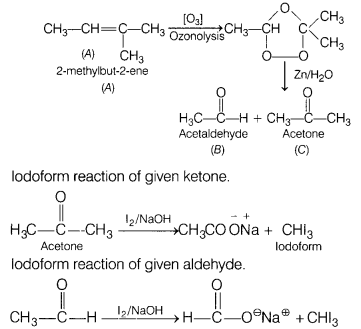
Or
(a) When liquid ‘A’ is treated with a freshly prepared ammoniacal silver
nitrate solution, it gives a bright silver mirror. The liquid forms a white
crystalline solid on treatment with sodium hydrogen sulphite.
Liquid ‘B’ also
forms a white crystalline solid with sodium hydrogen sulphite, but it does not
give a test with ammoniacal silver nitrate. (5)
(i) Identify A.
(ii)
Identify B.
(iii) Will boiling point of A and B be higher than their counter
hydrocarbon?
Answer:
(i) Liquid ‘A’ must be aldehyde because it reacts
with NaHSO3 and ammoniacal silver nitrate.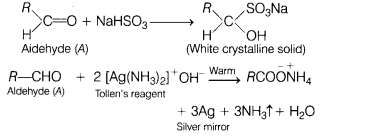
(ii) Liquid ‘B must be ketone because it does not react with ammoniacal
silver nitrate but reacts with NaHSO3.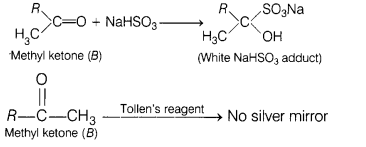
(iii) The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses. It is due to weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
(b) Draw the structures of the following ‘derivatives.
(i) Propanone
oxime
(ii) Semicarbazone of CH3CHO
Answer:
(i) Propanone
oxime The structure of propanone oxime is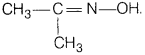
(ii) Semicarbazone of CH3CHO The structure of
semicarbazone of CH3CHO is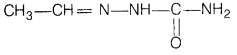
Question 32.
(a) What are fuel cells? Give an example of a fuel cell.
(b) Calculate the equilibrium constant (logKC) and ∆rG°
for the following reaction at 298 K.
Cu(s) + 2Ag+ (aq)
\(\rightleftharpoons\) Cu2+(aq) + 2Ag(s)
Given, E°cell
= 0.46 V and 1F = 96500 C mol-1
(c) Write the anode and cathode
reactions and the overall reaction occurring in a lead storage battery. (5)
Answer:
(a) Cell which converts energy of combustion of fuel directly into
electricity.
In other words we can say that fuel cells are those which
convert fuel energy directly into electrical energy, e.g. H2 –
O2 fuel cell.
(b)
(c)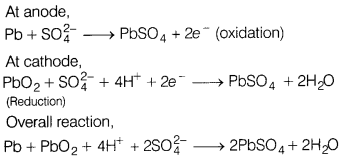
Or
(a) Write the cell reaction and calculate the emf of the following cell at
298 K.
Sn(s)|Sn2+(0.004 M) || H+(0.020
M)|H2(g) (1 bar) | Pt(s)
(Given : \(E^{\circ} \mathrm{Sn}^{2+} /
\mathrm{Sn}\) = -0.14 V)
(b) Give reasons.
(i) On the basis of E° values,
O2 gas should be liberated at anode, but it is Cl2 gas
which is liberated in the electrolysis of aqueous NaCl.
(ii) Conductivity of
CH3COOH decreases on dilution. (5)
Answer:
(a)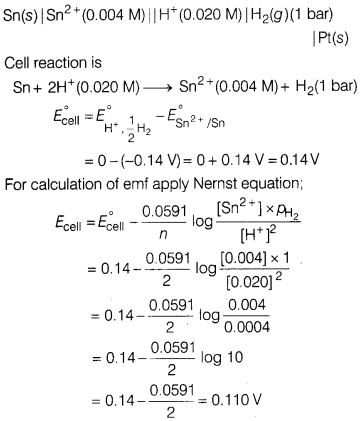
(b)
(i) From standard oxidising potential it is clear that oxygen gas
should be liberated at anode, but its rate of production is very low. In order
to increase that we increase the voltage of external battery because of which
chloride ions get oxidised easily and Cl2 gas is liberated at
anode.
(ii) Conductivity of solution is conductance of ions present in unit volume
of solution.
On dilution number of CH3COOH ions per unit volume
decreases. Hence, conductivity decreases.
Question 33.
Attempt any five of the following.
(a) Why do transition
elements show variable oxidation states?
(b) Generally there is an increase
in density of elements from titanium (Z = 22) to copper (Z = 29) in the first
series of transition elements.
(c) Transition elements and their compounds
are generally found to be good catalysts in chemical reactions.
(d) Why
copper cannot replace hydrogen from acids?
(e) Why first ionisation enthalpy
of Cr is lower than that of Zn?
(f) Mn3+ is a good oxidising
agent.
(g) \(E_{M^{2+} / M}^{\circ}\) values are not regular for first row
transition metals. (5)
Answer:
(a) Transition metals show a great variety
of oxidation states in its compounds (variable valency) except the first and the
last element. This is because of the fact that the difference in the energy of
(n – 1)d electrons and ns-electrons is low which implies that electrons from
both energy levels can takes part in bonding.
(b) From titanium to copper the atomic size of elements decreases and mass increases as a result of which density increases.
(c) The catalytic properties of the transition elements are due to the presence of unpaired electrons in their incomplete d-orbitals and variable oxidation states.
(d) The standard reduction potential of copper is positive (+0.34). Therefore, it cannot replace hydrogen from acids.
(e) The electronic configuration of chromium and zinc are
Cr(24)=[Ar]3d54s1 and Zn(30) =
[Ar]3d104s2
It is easy to remove electron from
4s1-orbital (unpaired electron) rather than from 4s2
orbital (paired electron). Thus, the first ionisation enthalpy of Cr is lower
than that of Zn.
(f) Mn3+ / Mn2+ has large positive E° value. Hence, Mn3+ can be easily reduced to Mn2+. Therefore, it is a good oxidising agent. Also, Mn2+ has half-filled electronic configuration, so it is more stable than Mn3+ state.
(g) There is decreasing negative electrode potentials of M2+/ M in the first transition series due to increase in the sum of IE1 and IE2. It shows that in general, the stability of +2 oxidation state decreases from left to right. Exceptions are Mn and Zn in which the greater stability of +2 state for Mn is due to half-filled d-subshell (d5) and that of Zn is due to completely tilled d-subshell (d10)