Atoms
Class 12th Physics Chapter Important Questions
Class 12 Physics Chapter 12 Important Extra Questions Atoms
Short Answer Type
Question 1.
Name the spectral series which lies in the visible region.
Answer:
Balmer series.
Question 2.
What is the maximum number of spectral lines emitted by a
hydrogen atom when it is in the third excited state? (CBSE AI 2013C)
Answer:
Six.
Question 3.
When is Hα line of the Balmer series in the
emission spectrum of hydrogen atom obtained? (CBSE Delhi 2013C)
Answer:
It
is obtained when an electron jumps from n =3 to n = 2 level.
Question 4.
A mass of lead is embedded in a block of wood. Radiations from
a radioactive source incident on the side of the block produce a shadow on a
fluorescent screen placed beyond the block. The shadow of the wood is faint but
the shadow of lead is dark. Give a reason for this difference.
Answer:
The
shadow of the wood is faint because only the a-radiations are stopped by the
wood (since a-radiations are least penetrating). The shadow of lead is dark
because p and y-radiations are also stopped by lead.
Question 5.
What was the source of alpha particles in Rutherford’s alpha
scattering experiment?
Answer:
The source was
21483Bi.
Question 6.
Define ionisation energy. What is its value for a hydrogen
atom? (All India 2010)
Answer:
Ionisation energy : The energy required to
knock out an electron from an atom is called ionisation energy of the atom.
For hydrogen atom it is 13.6 eV.
Question 7.
Write the expression for Bohr’s radius in hydrogen atom.
(Delhi 2010)
Answer:
Bohr’s radius in hydrogen atom,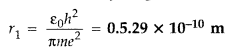
Question 8.
What is the ratio of radii of the orbits corresponding to
first excited state and ground state in a hydrogen atom? (Delhi 2010)
Answer:
Question 9.
The radius of innermost electron orbit of a hydrogen atom is
5.3 × 10-11 m. What is the radius of orbit in the second excited
state? (Delhi 2011)
Answer: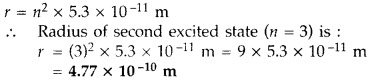
Question 10.
Find the ratio of energies of photons produced due to
transition of an electron of hydrogen atom from its
(i) second permitted
energy level to the first level, and
(ii) the highest permitted energy level
to the first permitted level. (All India 2010)
Answer: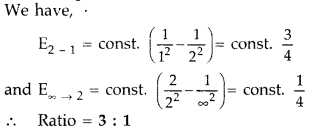
Question 11.
The ground state energy of hydrogen atom is -13.6 eV. What are
the kinetic and potential energies of electron in this state? (All India)
Answer:
Kinetic energy, Ke = + T.E. = 13.6 eV
Potential energy,
Pe = 2 T.E. = 2 (-13.6) = – 27.2 eV
Question 12.
Why is the classical (Rutherford) model for an atom—of
electron orbitting around the nucleus—not able to explain the atomic structure?
(All India 2012)
Answer:
As the revolving electron loses energy
continuously, it must spiral inwards and eventually fall into the nucleus. So it
was not able to explain the atomic structure.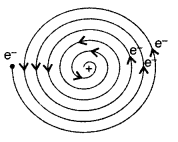
Question 13.
When is Ha line of the Balmer series in the emission spectrum
of hydrogen atom obtained? (Comptt. Delhi 2012)
Answer:
Balmer series is
obtained when an electron jumps to the second orbit (n1 = 2) from any
orbit n2 = n > 2 .
Question 14.
What is the maximum number of spectral lines emitted by a
hydrogen atom when it is in the third excited state? (Comptt. All India
2012)
Answer:
For third excited state, n2 = 4, and
n1 = 3, 2, 1 Hence there are 3 spectral lines.
Question 15.
If the radius of the ground level of a hydrogen atom is 5.3
nm, what is the radius of the first excited state?
Answer:
It is 4 × 5.3 =
21.2 nm ( ∵ r = n²ro)
Question 16.
Calculate the ratio of energies of photons produced due to the
transition of electron of a hydrogen atom from its
(a) Second permitted
energy level to the first level, and
Answer:
energy of photon
E1 = – 3.4 – (-13.6) = 10.2 eV
(b) Highest permitted energy level to the second permitted level.
Answer:
energy of photon E2 = 0 – (-3.4) = 3.4 eV
Ratio
\(\frac{E_{1}}{E_{2}}=\frac{10.2}{3.4}\) = 3
Question 17.
The mass of an H-atom is less than the sum of the masses of a
proton and electron. Why is this? (NCERT Exemplar) Answer:
Einstein’s
mass-energy equivalence gives E = mc². Thus the mass of an H-atom is
mp + me – B/c² where B ≈ 13.6 eV
Short Answer Type
Question 1.
(i) In hydrogen atom, an electron undergoes transition from
2nd excited state to the first excited state and then to the ground state.
Identify the spectral series to which these transitions belong.
(ii) Find out
the ratio of the wavelengths of the emitted radiations in the two cases.
(Comptt. All India 2012)
Answer:
Question 2.
(i) In hydrogen atom, an electron undergoes transition from
third excited state to the second excited state and then to the first excited
state. Identify the spectral series to which these transitions belong.
(ii)
Find out the ratio of the wavelengths of the emitted radiations in the two
cases. (Comptt. All India 2012)
Answer: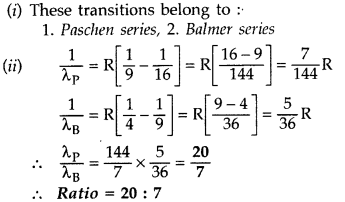
Question 3.
In hydrogen atom, an electron undergoes transition from 3rd
excited state to the first excited state and then to the ground state. Identify
the spectral series to which these transitions belong.
(ii) Find out the
ratio of the wavelengths of the emitted radiations in the two cases. (Comptt.
All India 2012) Answer:
(i) These transitions belong to :
1. Balmer
series,
2. Lyman series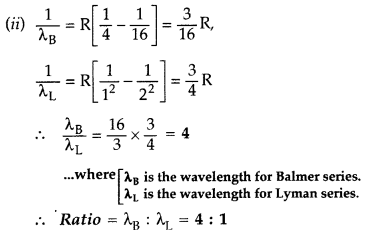
Question 4.
Using Rutherford model of the atom, derive the expression for
the total energy of the electron in hydrogen atom. What is the significance of
total negative energy possessed by the electron? (All India 2012)
Answer:
Expression for total energy of electron in H-atom using Rutherford model : As
per Rutherford model of atom, centripetal force (Fc) required to keep
electron revolving in orbit is provided by the electrostatic force (Fe) of
attraction between the revolving electron and nucleus.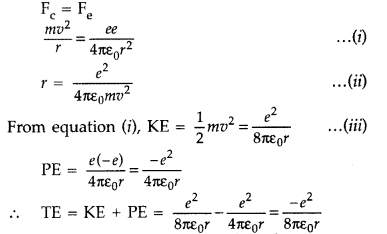
The negative sign indicates that the revolving electron is bound to the positive
nucleus.
Question 5.
Using Bohr’s postulates of the atomic model, derive the
expression for radius of nth electron orbit. Hence obtain the expression for
Bohr’s radius. (All India 2012)
Answer:
Basic postulates of Bohr’s atomic
model:
(i) Every atom consists of a central core called nucleus in which
entire positive charge and mass of the atom are concentrated. A suitable number
of electrons revolve around the nucleus in circular orbit. The centripetal force
required for revolution is provided by the electrostatic force of attraction
between the electron and the nucleus.
(ii) Electron can resolve only in
certain discrete non-radiating orbits, called stationary orbit. Total angular
momentum of the revolving electron in an integral multiple of h/2π.
… where
[h is plank constant]![]()
(iii) The radiation of energy occurs only when an electron
jumps from one permitted orbit to another. The difference in the total energy of
electron in the two permitted orbit is absorbed when the electron jumps from
inner to the outer orbit and emitted when electron jumps from outer to inner
orbit.
Radii of Bohr’s stationary orbits. According to Bohr’s postulates, angular
momentum of electron for any permitted orbit is,![]()
Also, according to Bohr’s postulates, the centripetal force is equal to
electrostatic force between the electron and nucleus.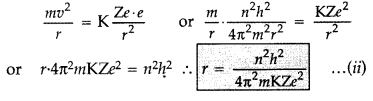
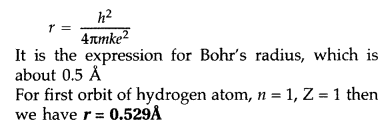
Question 6.
Show that the radius of the orbit in hydrogen atom varies as
n2, where n is the principal quantum number of the atom. (Delhi
2012)
Answer:
When an electron moves around hydrogen nucleus, the
electrostatic force between electron and hydrogen nucleus provides necessary
centrepetal force.![]()
Also we know from Bohr’s postulate,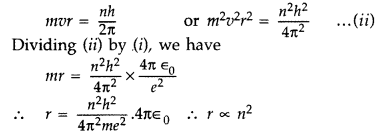
Question 7.
When an electron in hydrogen atom jumps from the third
excited state to the ground state, how would the de Broglie wavelength
associated with the electron change? Justify your answer. (All India 2012)
Answer: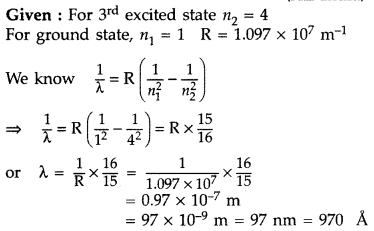
It lies in the ultra-violet region.
Question 8.
Calculate the shortest wavelength in the Balmer series of
hydrogen atom. In which region (infra-red, visible, ultraviolet) of hydrogen
spectrum does this wavelength lie? (All India 2012)
Answer:
In Balmer
series, an electron jumps from higher orbits to the second stationary orbit
(nf = 2). Thus for this series :
Question 9.
The figure shows energy level diagram of hydogen atom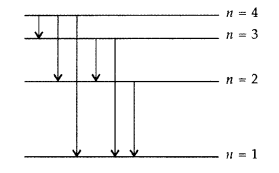
(a) Find out the transition which results in the emission of
a photon of wavelength 496 nm.
(b) Which transition corresponds to the
emission of radiation of maximum wavelength? Justify your answer. (Comptt. All
India 2012)
Answer:
(a) Transition emitting wavelength λ = 496 nm The
given wavelength lies in visible region (Balmer series) when,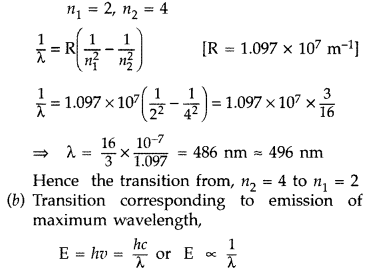
which means that the maximum wavelength emmission will be there when the energy
level difference is minimum. From the given energy level diagram, it corresponds
to :![]()
Question 10.
In Rutherford scattering experiment, draw the trajectory
traced by a-particles in the coulomb field of target nucleus and explain how
this led to estimate the size of the nucleus. (Comptt. All India 2012)
Answer:
Note: The Rutherford scattering experiment is also known as the
Geiger Marsden experiment.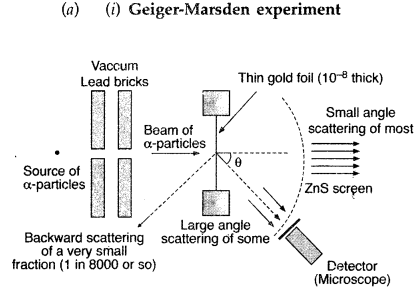
(ii) For most of the α-particles, impact parameter is large, hence they suffer
very small repulsion due to nucleus and go right through the foil.
(iii)
Trajectory of α-particles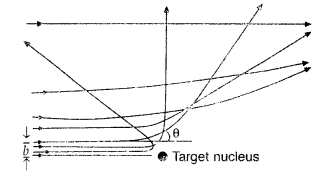
It gives an estimate of the size of nucleus, that it relatively very very small
as compared to the size of atom.
Question 11.
Define ionization energy.
How would the ionization energy
change when electron in hydrogen atom is replaced by a particle of mass 200
times that of the electron but having the same charge? (All India 2016)
Answer:
Definition of ionization energy : “The minimum energy, required to
free the electron from the ground state of the hydrogen atom, is known as
Ionization Energy.”
The ionization energy is given by :![]()
∴Ionization Energy will become 200 times,
∵ the mass of
given particle is 200 times.
Question 12.
Calculate the shortest wavelength of the spectral lines
emitted in Balmer series.
[Given Rydberg constant, R = 107
m-1] (All India 2016)
Answer: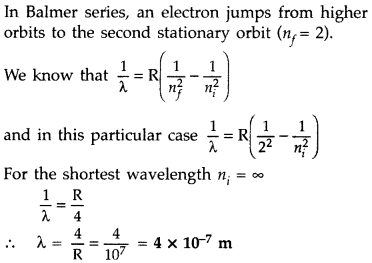
Question 13.
The electron, in a hydrogen atom, is in its second excited
state.
Calculate the wavelength of the lines in the Lyman series, that can be
emitted through the permissible transitions of this electron.
(Given the
value of Rydberg constant, R = 1.1 × 107 m-1) (Comptt. Delhi
2016)
Answer: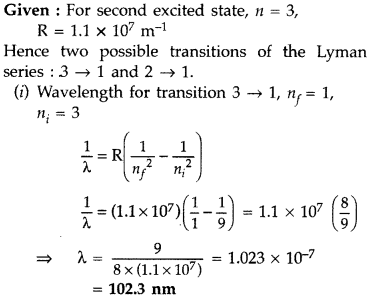
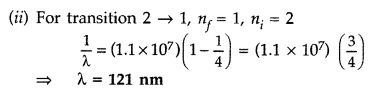
Question 14.
An α-particle moving with initial kinetic energy K towards a
nucleus of atomic number z approaches a distance ‘d’ at which it reverses its
direction. Obtain the expression for the distance of closest approach ‘d’ in
terms of the kinetic energy of α-particle K. (Comptt. All India 2016)
Answer: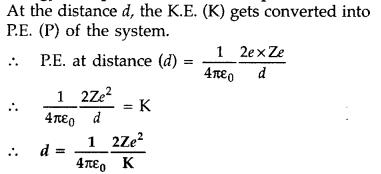
Question 15.
Find the ratio between the wavelengths of the ‘most
energetic’ spectral lines in the Balmer and Paschen series of the hydrogen
spectrum. (Comptt. All India 2016)
Answer: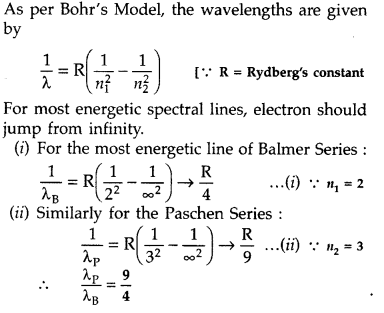
Question 16.
Define the distance of closest approach. An a-particle of
kinetic energy ‘K’ is bombarded on a thin gold foil. The distance of the closest
approach is V. What will be the distance of closest approach for an a-particle
of double the kinetic energy? (Delhi 2016)
Answer:
The distance of closest
approach is defined as “the distance of charged particle from the centre of the
nucleus, at which the whole of the initial kinetic energy of the (far off)
charged particle gets converted into the electric potential energy of the
system”.
Distance of closest approach (rc) is given by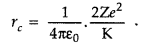
Question 17.
Write two important limitations of Rutherford nuclear model
of the atom. (Delhi 2016)
Answer:
Important limitations of Rutherford
Model :
- According to Rutherford model, electron orbiting around the nucleus, continuously radiates energy due to the acceleration; hence the atom will not remain stable.
- As electron spirals inwards; its angular velocity and frequency change continuously; therefore it. will emit a continuous spectrum.
Question 18.
Find out the wavelength of the electron orbiting in the
ground state of hydrogen atom. (Delhi 2016)
Answer: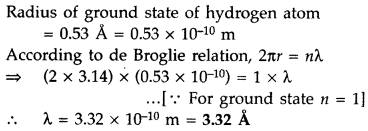
Question 19.
Find the wavelength of the electron orbiting in the first
excited state in hydrogen atom. (Delhi 2016)
Answer: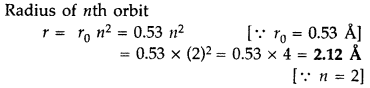
Question 20.
A 12.5 eV electron beam is used to excite a gaseous hydrogen
atom at room temperature. Determine the wavelengths and the corresponding series
of the lines emitted. (All India 2016)
Answer: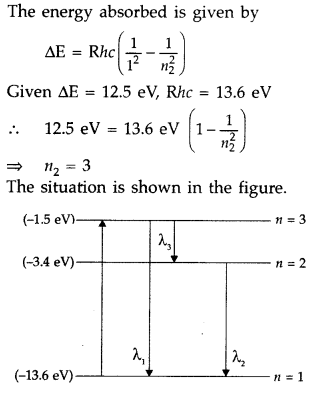
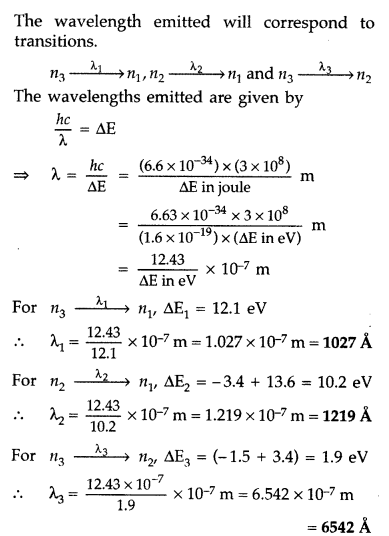
Question 21.
The short wavelength limit for the Lyman series of the
hydrogen spectrum is 913.4 A Calculate the short wavelength limit for Balmer
series of the hydrogen spectrum. (All India 2016)
Answer: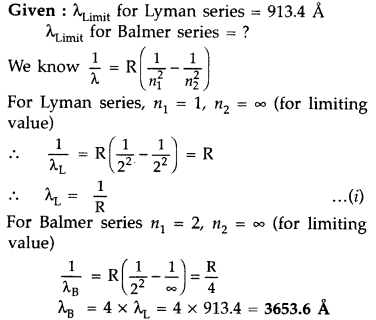
Question 22.
The ground state energy of hydrogen atom is -13.6 eV. If an
electron makes a transition from an energy level -1.51 eV to -3.4 eV, calculate
the wavelength of the spectral line emitted and name the series of hydrogen
spectrum to which it belongs. (All India 2016)
Answer:![]()
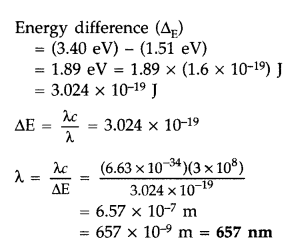
Question 23.
Calculate the shortest wavelength of light emitted in the
Paschen series of hydrogen spectrum. Which part of the electromagnetic spectrum,
does it belong to ? (Given : Rydberg constant, R = 1.1 × 107
m-1) (Comptt. Delhi 2016)
Answer: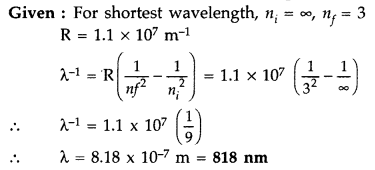
It belongs to the Infrared part of the spectrum.
Question 24.
Calculate the shortest wavelength of photons emitted in the
Bracket series of hydrogen spectrum. Which part of the e.m. spectrum, does it
belong? [Given Rydberg constant, R = 1.1 × 107 m-1]
(Comptt. Delhi 2016)
Answer:
In bracket series, nf = 4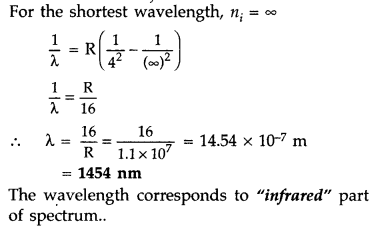
Question 25.
Calculate the longest wavelength of the photons emitted in
the Balmer series of hydrogen spectrum. Which part of the e.m. spectrum, does it
belong to?
[Given Rydberg constant, R = 1.1 × 107 m-1]. (Comptt.
Delhi 2016)
Answer:
Balmer series is produced when an electron jumps from
higher orbits to second stationary orbit (nf = 2).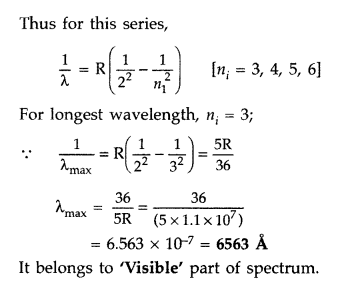
Question 26.
Define electron-volt and atomic mass unit. Calculate the
energy in joule equivalent to the mass of one proton.
Answer:
Electron
volt: It is defined as the energy gained by an electron when accelerated through
a potential difference of 1 volt. Atomic mass unit: It is defined as one-twelfth
of the mass of one atom of carbon 12.
The mass of a proton is 1.67 × 10-27 kg. Therefore, energy equivalent of this mass is E = mc² = 1.67 × 10-27 × (3 × 108)2 = 1.5 × 10-10 J
Question 27.
State Bohr’s quantization condition of angular momentum.
Calculate the shortest wavelength of the Bracket series and state to which part
of the electromagnetic spectrum does it belong. (CBSE Delhi 2019)
Or
Calculate the orbital period of the electron in the first excited state of the
hydrogen atom.
Answer:
Bohr’s Quantisation condition: Only those orbits
are permitted in which the angular momentum of the electron is an integral
multiple of h/2π.
For Brackett Series,
The shortest wavelength is for the transition of
electrons from ni = ∞ to nf = 4
Using the equation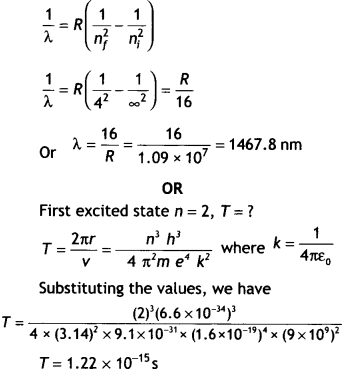
Question 28.
Write two important limitations of the Rutherford nuclear model
of the atom. (CBSE AI2018, Delhi 2018)
Answer:
- Rutherford’s model fails to explain the line spectra of the atom.
- Rutherford’s model cannot explain the stability of the nucleus.
Question 29.
Find out the wavelength of the electron orbiting in the ground
state of the hydrogen atom. (CBSEAI 2018, Delhi 2018)
Answer:
The
wavelength of an electron in the ground state of hydrogen atom is given by
E
= \(\frac{hc}{λ}\)
or
λ = \(\frac{hc}{E}\)
For ground state
E = – 13.6 eV = 13.6 × 1.6 × 10-19 J
Hence wavelength of electron in the first orbit
λ = \(\frac{h
c}{E}=\frac{6.6 \times 10^{-34} \times 3 \times 10^{8}}{13.6 \times 1.6 \times
10^{-19}}\) = 0.9 × 10-7 J
Question 30.
(a) State Bohr’s postulate to define stable orbits in a
hydrogen atom. How does de Broglie’s hypothesis explain the stability of these
orbits?
Answer:
Bohr’s postulate for stable orbits states the electron in
an atom revolves around the nucleus only in those orbits for which its angular
momentum is an integral multiple of h/2π (h = Planck’s constant), (n = 1, 2, 3
…)
As per de Broglie’s hypothesis λ = h/p = h/mv
For a stable orbit, we must
have a circumference of the orbit = nλ (n = 1, 2, 3,…)
∴ 2πr = nλ
or
mvr = nh/2π
Thus de-Broglie showed that the formation . of stationary patterns for
integral “n” gives rise to the stability of the atom.
This is nothing but
Bohr’s postulate.
(b) A hydrogen atom initially in the ground state absorbs a photon which
excites it to the n = 4 level. Estimate the frequency of the photon.
Answer:
Energy in the n = 4 level n1 = 1 and
n2 = 4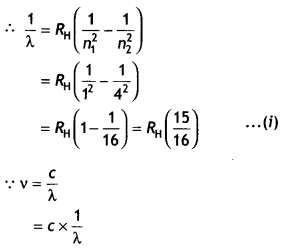
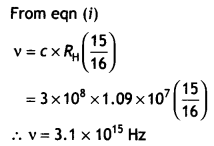
Question 31.
An alpha particle moving with initial kinetic energy K towards a
nucleus of atomic number Z approaches a distance ‘d’ at which it reverses its
direction. Obtain an expression for the distance of closest approach ‘d’ in
terms of the kinetic energy of the alpha particle, K. (CBSEAI2016C)
Answer:
At the distance of the closest approach, the kinetic energy of the
alpha particle is converted into the electrostatic potential energy of the alpha
particle-nucleus system. Therefore, at the distance of the closest approach
we have
Kinetic energy = Potential energy
Therefore,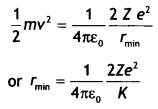
where K is the kinetic energy.
Question 32.
The figure shows the energy level diagram of the hydrogen
atom.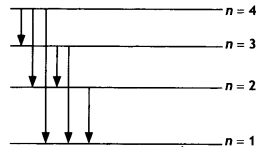
(a) Find out the transition which results in the emission of a photon of
wavelength 496 nm.
Answer:
The wavelength of photon emitted is given by
\(\frac{1}{λ}\) =
RH\(\left(\frac{1}{n_{\mathrm{f}}^{2}}-\frac{1}{n_{\mathrm{i}}^{2}}\right)\)
None of these transitions correspond to a wavelength of 496 nm. The closest is 4 to 2 of 489 nm
(b) Which transition corresponds to the emission of radiation of maximum
wavelength? Justify your answer. (CBSE AI 2015 C)
Answer:
Transition 4 to
3 as the frequency of this radiation is maximum.
Question 33.
A nucleus makes a transition from one permitted energy level
to another level of lower energy. Name the region of the electromagnetic
spectrum to which the emitted photon belongs. What is the order of its energy in
electron-volts? Write four characteristics of nuclear forces.
Answer:
(a)
Emitted photon belongs to gamma-rays part of the electromagnetic spectrum.
(b) the energy is of the order of MeV.
(c) Four characteristics of nuclear
forces are:
- Nuclear forces are independent of charges.
- Nuclear forces are short-range forces.
- Nuclear forces are the strongest forces in nature, in their own small range of few fermis.
- Nuclear forces are saturated forces.
Question 34.
The energy level diagram of an element is given. Identify, by
doing necessary calculations, which transition corresponds to the emission of a
spectral line of wavelength 102.7 nm. (Delhi 2008)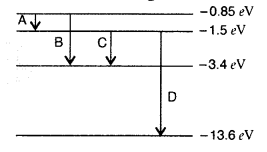
Answer: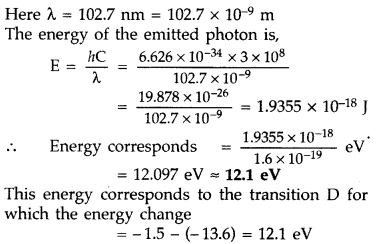
Question 35.
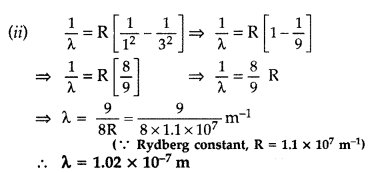
The ground state energy of hydrogen atom is -13.6 eV.
(i)
What is the kinetic energy of an electron in the 2nd excited
state?
(ii) If the electron jumps to the ground state from the 2nd
excited state, calculate the wavelength of the spectral line emitted. (All India
2008)
Answer: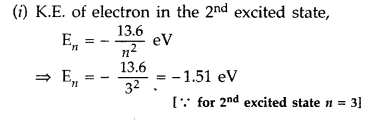
Question 36.
The ground state energy of hydrogen atom is -13.6 eV.
(i)
What is the potential energy of an electron in the 3rd excited
state?
(ii) If the electron jumps to the ground state from the 3rd
excited state, calculate the wavelength of the photon emitted. (All India
2008)
Answer: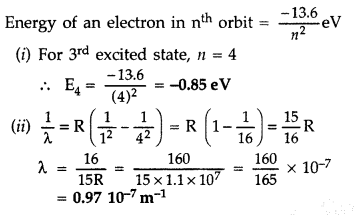
Question 37.
(a) The energy levels of an atom are as shown here. Which of
them will result in the transition of a photon of wavelength 275 nm?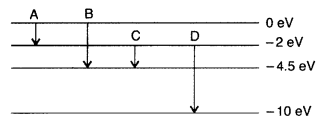
(b) Which transition corresponds to emission of radiation of maximum wavelength?
(Delhi 2008)
Answer: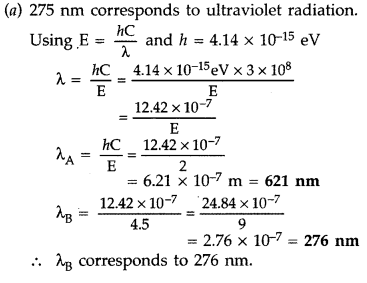
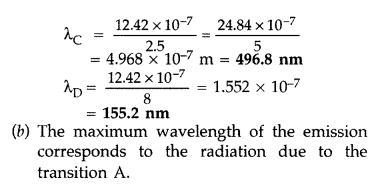
Question 38.
The energy levels of a hypothetical atom are shown below.
Which of the shown transitions will result in the emission of a photon of
wavelength 275 nm? Which of these transitions correspond to emission of
radiation of
(i) maximum and
(ii) minimum wavelength ?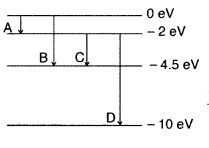
(Delhi 2011)
Answer: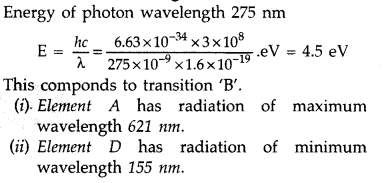
Question 39.
(a) Using Bohr’s second postulate of quantization of orbital
angular momentum show that the circumference of the electron in the n,h orbital
state in hydrogen atom is n times the de-Broglie wavelength associated with
it.
(b) The electron in hydrogen atom is initially in the third excited
state. What is the maximum number of spectral lines which can be emitted when it
finally moves to the ground state? (Delhi 2012)
Answer:
(a) According to
the de-Broglie hypothesis, this electron is also associated with wave
character.
Hence a circular orbit can be taken to be a stationary energy
state only if it contains an integral number of de- Broglie wavelengths i.e. we
must have
2πr = nλ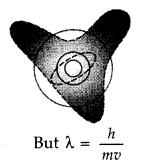
According to Bohr’s-second postulate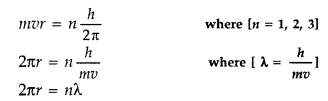
Hence the circumference of the electron in the nth orbital state in
hydrogen atom is n times the de-Broglie wavelength associated with it.
(b) For third excited state n = 4
For ground state n = 1
Hence,
possible transitions are :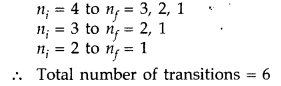
Question 40.
In a Geiger-Marsden experiment, calculate the distance of
closest approach to the nucleus of Z = 80, when an α-particle of 8 MeV energy
impinges on it before it comes momentarily to rest and reverses its
direction.
How will the distance of closest approach be affected when the
kinetic energy of the α-particle is doubled? (All India 2012)
Answer: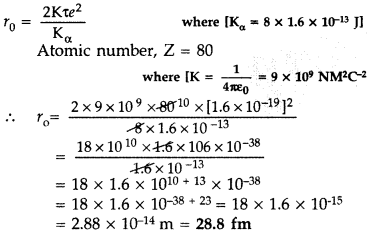
When kinetic energy of a-particle is doubled, then distance of closest approach
becomes half
Question 41.
The ground state energy of hydrogen atom is -13.6 eV. If an
electron makes a transition from an energy level -0.85 eV to -3.4 eV, calculate
the wavelength of the spectral line emitted. To which series of hydrogen
spectrum does this wavelength belong? (All India 2012)
Answer: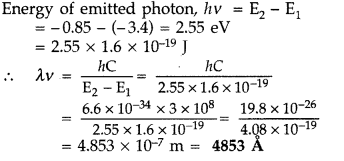
(ii) This wavelength belongs to Balmer series of hydrogen spectrum.
Question 42.
Using Bohr’s postulates, obtain the expression for the total
energy of the electron in the stationary states of the hydrogen atom. Hence draw
the energy level diagram showing how the line spectra corresponding to Balmer
series occur due to transition between energy levels. (Delhi 2012)
Answer: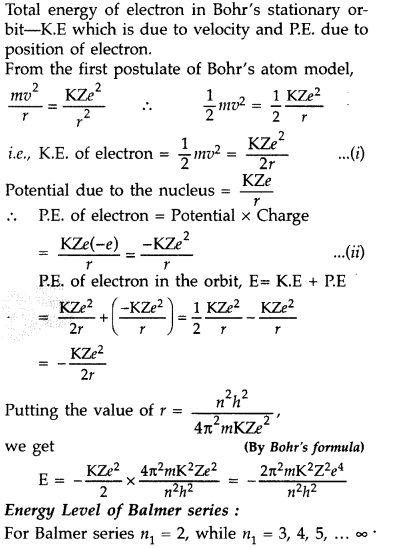
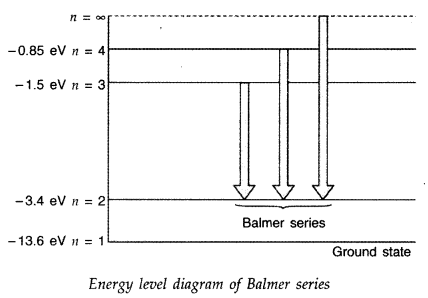
Question 43.
Using Bohr’s postulates, obtain the expressions for
(i)
kinetic energy and
(ii) potential energy of the electron in stationary state
of hydrogen atom. Draw the energy level diagram showing how the transitions
between energy levels result in the appearance of Lyman Series. (Delhi 2012)
Answer: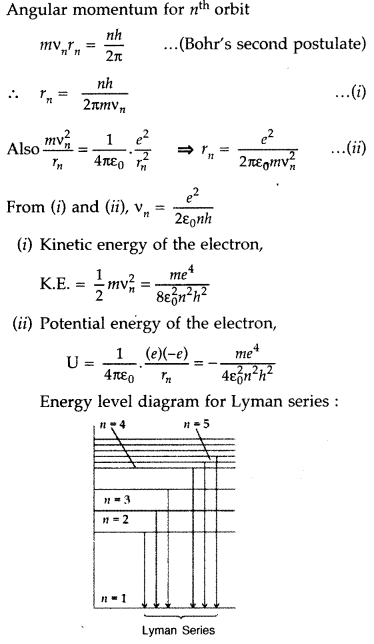
Question 44.
In the ground state of hydrogen atom, its Bohr radius is
given as 5.3 × 10-11 m. The atom is excited such that the radius
becomes 21.2 × 10-11 m. Find
(i) the value of the principal
quantum number and
(ii) the total energy of the atom in this excited state.
(Comptt. Delhi 2012)
Answer: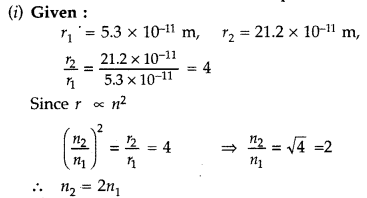
Therefore, Value of principal quantum number in this excited state is 2.
(ii)
Energy of electron in ground state = -13.6 eV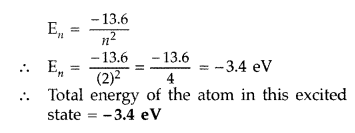
Question 45.
A 12.5 eV electron beam is used to bombard gaseous hydrogen
at room temperature. Upto which energy level the hydrogen atoms would be
excited? Calculate the wavelengths of the first member of Lyman and first member
of Balmer series. (Delhi 2014)
Answer: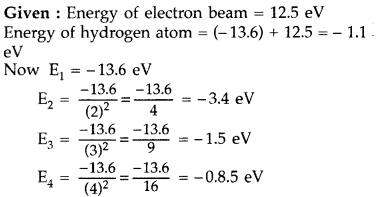
Hydrogen atom will be excited upto third energy level, because energy of
electron is less than that of 4th energy level.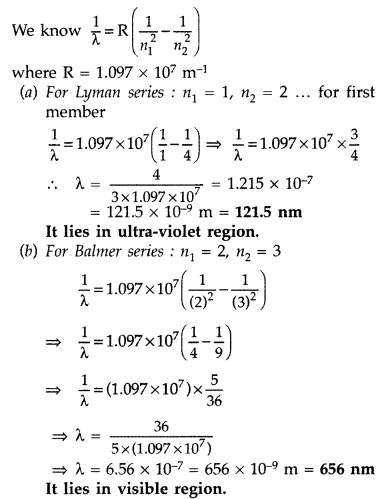
Question 46.
A 12.9 eV beam of electrons is used to bombard gaseous
hydrogen at room temperature. Upto which energy level the hydrogen atoms would
be excited?
Calculate the wavelength of the first member of Paschen series
and first member of Balmer series. (Delhi 2014)
Answer:
1st part: Similar
to Q. 46, Page 280
Wavelength of the first member of Paschen series:
n1 = 3, n2 = 4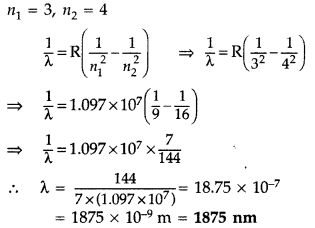
It lies in infra-red region.
Question 47.
A 12.3 eV electron beam is used to bombard gaseous hydrogen
at room temperature. Upto which energy level the hydrogen atoms would be
excited?
Calculate the wavelengths of the second member of Lyman series and
second member of Balmer series. (Delhi 2014)
Answer:
1st part: Similar to
Q. 46, Page 280
(i) Wavelength of second member of Lyman series :
n1 = 1, n2 = 3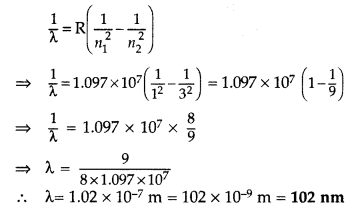
∴ It lies in ultra violet region.
(ii) Wavelength of second member of Balmer
series (n1 = 2, n2 = 4)
It lies in visible region.
Question 48.
(a) The radius of the innermost electron orbit of a hydrogen
atom is 5.3 × 10-11 m. Calculate its radius in n = 3 orbit.
(b)
The total energy of an electron in the first excited state of the hydrogen atom
is -3.4 eV. Find out its
(i) kinetic energy and
(ii) potential energy in
this state. (Comptt. Delhi 2014)
Answer: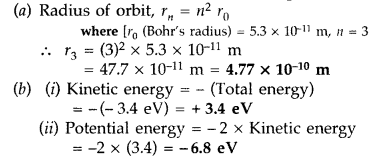
Question 49.
(a) The radius of the innermost electron orbit of a hydrogen
atom is 5.3 × 1011 m. Calculate its radius in n = 2 orbit.
(b) The
total energy of an electron in the second excited state of the hydrogen atom is
-1.51 eV. Find out its
(i) kinetic energy and
(ii) potential energy in
this state. (Comptt. Delhi 2014)
Answer: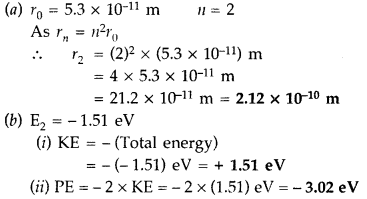
Question 50.
(a) The orbital radius of the electron in the first excited
state of hydrogen atom is 21.2 × 10-11 m. Find out its radius in the
second excited state.
(b) The total energy of the electron in the ground
state is -13.6 eV. Find out
(i) its. kinetic energy and
(ii) potential
energy in the first excited state. (Comptt. Delhi 2014)
Answer: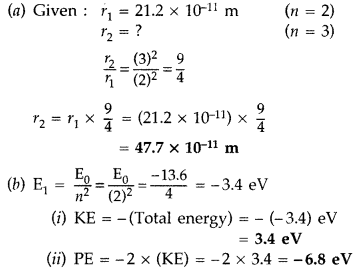
Question 51.
The value of a ground state energy of hydrogen atom is -13.6
eV.
(i) Find the energy required to move an electron from the ground state to
the first excited state of the atom.
(ii) Determine
(a) the kinetic energy
and
(b) orbital radius in the first excited state of the atom. (Given the
value of Bohr radius = 0.53 Å) (Comptt. All India 2014)
Answer: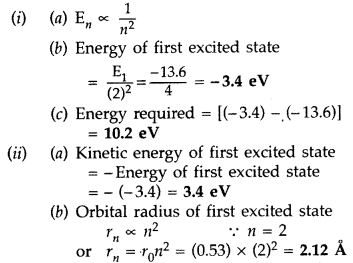
Question 52.
The value of ground state energy of hydogen atom is -13.6 eV
and Bohr radius is 0.53Å. Calculate
(i) the energy required to move an
electron from the ground state to the second excited state.
(ii) (a) the
kinetic energy and
(b) the orbital radius in the second excited state of the
atom. (Comptt. All India 2014)
Answer: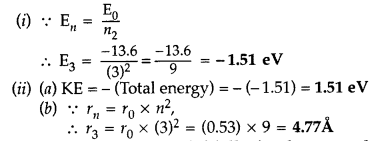
Question 53.
(i) A hydrogen atom initially in the ground state absorbs a
photon which excites it to the n- 4 level. Determine the wavelength of the
photon.
(ii) The radius of innermost electron orbit of a hydrogen atom is 5.3
× 10-11m. Determine its radius in n = 4 orbit.(Comptt. All India
2014)
Answer: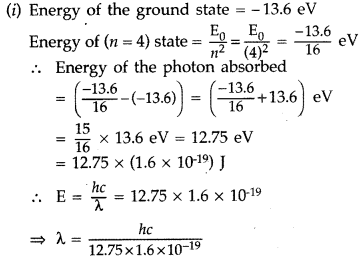
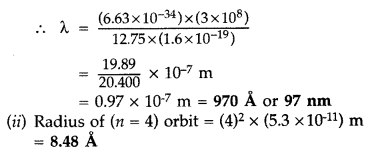
Question 54.
In the study of Geiger-Marsdon experiment on scattering of a
particles by a thin foil of gold, draw the trajectory of a-particles in the
coulomb field of target nucleus. Explain briefly how one gets the information on
the size of the nucleus from this study.
From the relation, R = R0
\(\mathbf{A}^{1 / 3}\), where R0, is constant and A is the mass
number of the nucleus, show that nuclear matter density is independent of A.
(Delhi 2015)
Answer:
(i) Trajectory of α-particles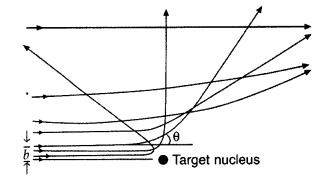
(ii) Size of nucleus : Only a small fraction of the incident a-particles
rebound. This shows that the mass of the atom is concentrated in a small volume
in the form of nucleus and gives an idea of the size of nucleus.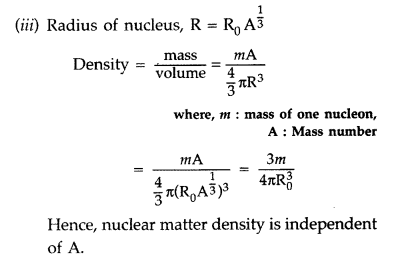
Question 55.
(i) State Bohr’s quantization condition for defining
stationary orbits. How does de-Broglie hypothesis explain the stationary
orbits?
(ii) Find the relation between the three wave-lengths λ1,
λ2 and λ3 from the energy level diagram shown below:
(Delhi 2016)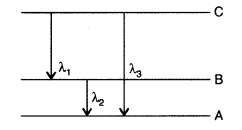
Answer: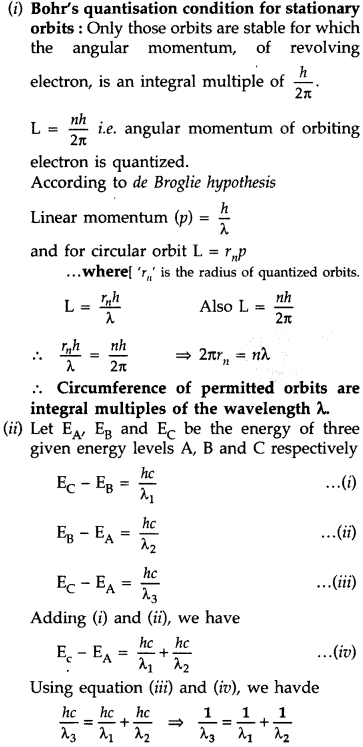
Question 56.
Using Bohr’s postulates, derive the expression for the total
energy of the electron revolving in nth orbit of hydrogen atom. Find
the wavelength of H line, given value of Ryderg constant, R = 1.1 ×
107m-1.
Answer:
For total energy of the electron
orbiting in nth orbit :
Total energy of electron in Bohr’s
stationary orbit K.E. which is due to velocity and P.E. due to position of
electron
From the first postulate of Bohr’s atom modal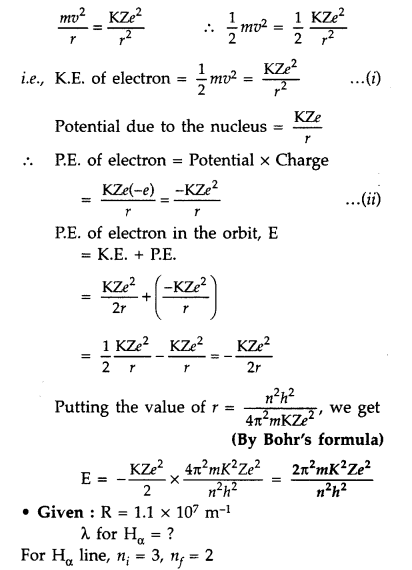
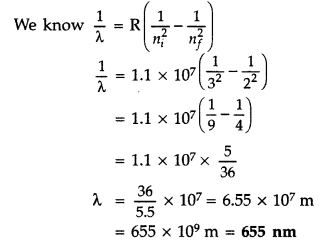
Long Answer Type
Question 1.
Explain Rutherford’s experiment on the scattering of alpha
particles and state the significance of the results.
Answer:
The schematic
arrangement in the Geiger Marsden experiment is shown in the figure.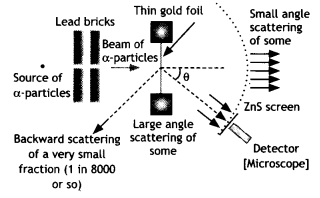
Alpha-particles emitted by a Bismuth (21483Bi) radioactive
source were collimated into a narrow beam by their passage through lead bricks.
The beam was allowed to fall on a thin foil of gold of thickness 2.1 ×
10-7 m. The scattered alpha-particles were observed through a
rotatable detector consisting of a zinc sulfide screen and a microscope. The
scattered alpha-particles on striking the screen produced bright light flashes
or scintillations. These scintillations could be viewed through the microscope
and counted at different angles from the direction of the incident beam.
Significance: The experiment established the existence of a nucleus that contained the entire positive charge and about 99.95% of the mass.
Question 2.
Using Bohr’s postulates, obtain the expression for the total
energy of the electron in the stationary states of the hydrogen atom. Hence draw
the energy level diagram showing how the line spectra corresponding to the
Balmer series occur due to the transition between energy levels. (CBSE Delhi
2013)
Answer:
The electron revolving around the nucleus has two types of
energy:
Kinetic energy due to its motion.
Potential energy due to it lying in the
electric field of the nucleus.
Thus the total energy of the electron is given by
E = K. E. + P. E.
…(1)
An electron of mass m moving around the nucleus with an orbital velocity v
has kinetic energy given by
K.E. = \(\frac{1}{2}\)mv² = \(\frac{1}{2} \frac{k
e^{2}}{r}\) …(2)
Now the potential energy of the electron at a distance r from the nucleus is
given by
PE = potential due to the nucleus at a distance r × charge on the
electron = V × – e …(3)
Now the potential at a distance r from the nucleus having a charge e is given
by
V = k \(\frac{e}{r}\) …..(4)
Substituting in equation (3) we have
P.E. = V × – e =
-k\(\frac{e^{2}}{r}\) …(5)
Substituting equations (2) and (3) in equation 1 we have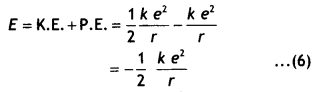
But the radius of the nth orbit is given by
rn = \(\frac{n^{2}
h^{2}}{4 \pi^{2} m e^{2} k}\)
Substituting in equation (6) we have
E = – \(\frac{2 \pi^{2} m e^{4}
\mathrm{k}^{2}}{n^{2} \mathrm{~h}^{2}}\) …(7)
This gives the expression for the energy possessed by the electron in the nth orbit of the hydrogen atom.
Question 3.
Hydrogen atoms are excited with an electron beam of energy of
12.5 eV. Find
(a) The highest energy level up to which the hydrogen atoms
will be excited.
Answer:
The maximum energy that the excited hydrogen atom
can have is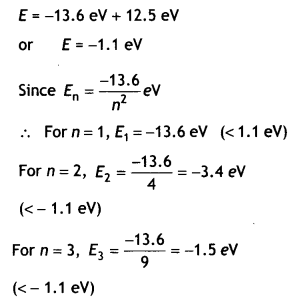
For n=4, E4 = \(\frac{-13.6}{16}\) = – 0.85eV
(> – 1.1 eV)
∴ The eLectron can only be excited up to n = 3 states.
(b) The longest wavelengths in the (i) Lyman series, (ii) Balmer series of
the spectrum of these hydrogen atoms. (CBSE 2019C)
Answer:
From energy
tevet of hydrogen atom,
we have
\(\frac{1}{λ}\) =
R\(\left[\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}}\right]\)
Longest wavelength of Lyman senes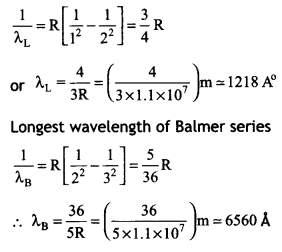
Question 4.
Using Bohr’s postulates of the atomic model derive the expression
for the radius of the 11th electron orbit. Hence obtain the
expression for Bohr’s radius. (CBSE AI 2014)
Answer:
Let us consider a
mechanical model of the hydrogen atom as shown in the figure that incorporates
this quantization assumption.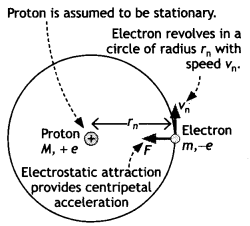
This atom consists of a single electron with mass m and charge – e revolving
around a single proton of charge + e. The proton is nearly 2000 times as massive
as the electron, so we can assume that the proton does not move. As the electron
revolves around the nucleus the electrostatic force of attraction between the
electron and the proton provides the necessary centripetal force. Therefore, we
have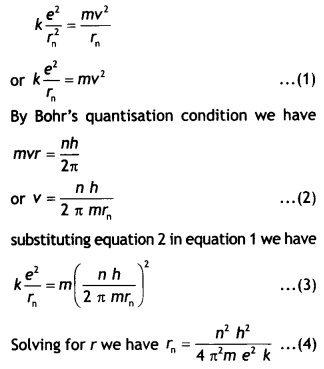
This gives the radius of the nth orbit of the hydrogen atom.
If n = 1 we have
r = ao which is called Bohr’s radius.
ao =
\(\frac{h^{2}}{4 \pi^{2} m e^{2} k}\)
Question 5.
Draw a schematic arrangement of the Geiger-Marsden
experiment. How did the scattering of α-particles by a thin foil of gold provide
an important way to determine an upper limit on the size of the nucleus? Explain
briefly.
(All India 2009)
Answer: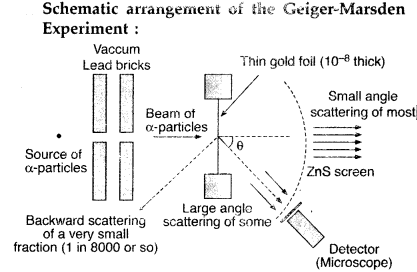
(i) Beam of α-particles get deviated at various angles with different
probabilities.
(ii) α-particles with least impact parameter suffers larger
scattering – rebounding on head on collision.
(iii) For larger impact
parameter, the particle remains almost undeviated.
Explanation:
The fact that the number of incident particles rebounding
back is only a small of fraction, means that numer of α-particles headon
collision is small. This implies that the entire positive charge of the atom is
concentrated in a small volume. This confirms that the nucleus of the atom has
an apper size limit.
Question 6.
(a) Using Bohr’s theory of hydrogen atom, derive the
expression for the total energy of the electron in the stationary states of the
atom.
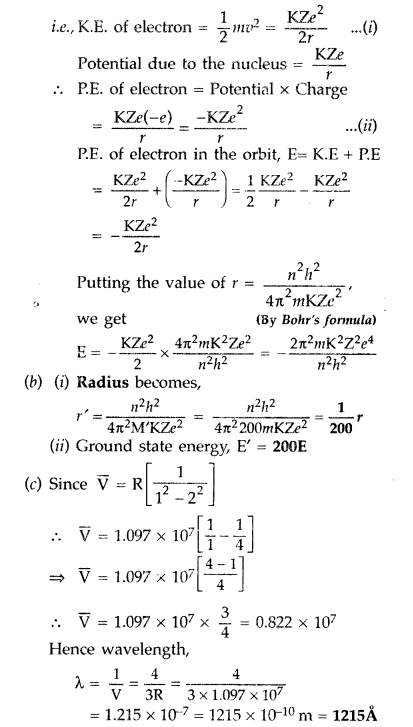
(b) If electron in the atom is replaced by a particle (muon) having the
same charge but mass about 200 times as that of the electron to form a muonic
atom, how would
(i) the radius and
(ii) the ground state energy of this be
affected?
(c) Calculate the wavelength of the first spectral line in the
corresponding Lyman series of this atom. (Comptt. Delhi 2012)
Answer:
(a)
Total energy of electron in Bohr’s stationary orbit—K.E which is due to velocity
and P.E. due to position of electron.
From the first postulate of Bohr’s atom
model,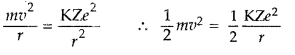
Question 7.
Using Bohr’s postulates, derive the expression for the
frequency of radiation emitted when electron in hydrogen atom undergoes
transition from higher energy state (quantum number ni) to the lower
state (nf).
When electron in hydrogen atom jumps from energy state
ni = 4 to nf = 3, 2, 1, identify the spectral series to
which the emission lines belong. (All India 2013)
Answer:
When a hydrogen
atom receives energy by process such as electron collisions of heat, the atom
may acquire sufficient energy to raise the electron to higher energy states from
n = 1 to n = 2, it is said to be in an excited state. When an electron falls
back from excited state to lower energy state by emitting, there is a photon of
particular energy and of particular frequency.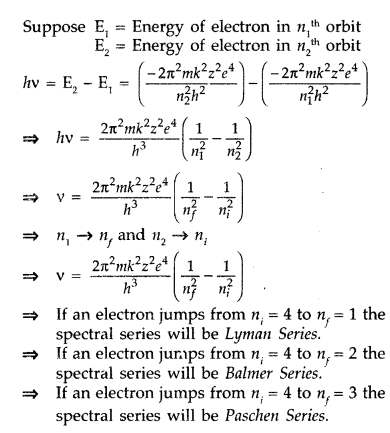
Question 8.
(a) Draw a schematic arrangement of Geiger- Marsden
experiment showing the scattering of a-particles by a thin foil of gold. Why is
it that most of the a-particles go right through the foil and only a small
fraction gets scattered at large angles?
Draw the trajectory of the
a-particle in the coulomb field of a nucleus. What is the significance of impact
parameter and what information can be obtained regarding the size of the
nucleus?
(b) Estimate the distance of closest approach to the nucleus (Z =
80) if a 7.7 MeV a-particle before it comes momentarily to rest and reverses its
direction. (Comptt. Delhi 2015)
Answer:
(ii) For most of the α-particles, impact parameter is large, hence they suffer
very small repulsion due to nucleus and go right through the foil.
(iii)
Trajectory of α-particles
It gives an estimate of the size of nucleus, that it relatively very very small
as compared to the size of atom.
(b) K.E. of the a-particle = Potential energy possessed by beam at distance
of closest approach.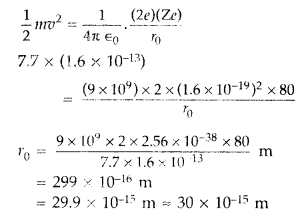
Question 9.
(a) Write two important limitations of Rutherford model which
could not explain the observed features of atomic spectra. How were these
explained in Bohr’s model. of hydrogen atom?
Use the Rydberg formula to
calculate the wavelength of the Hα line.
(Take R = 1.1 ×
107 m-1).
(b) Using Bohr’s postulates, obtain the
expression for the radius of the nth orbit in hydrogen atom. (Comptt.
Delhi 2015)
Answer:
(a) Limitations of Rutherford Model :
(i) Electrons
moving in a circular orbit around the nucleus would get, accelerated, therefore
it would spiral into the nucleus, as it looses its energy.
(ii) It must emit
a continuous spectrum.
Explanation according to Bohr’s model of hydrogen atom :
(i) Electron in an atom can revolve in certain stable orbits without the
emission of radiant energy.
(ii) Energy is released/absorbed only, when an
electron jumps from one stable orbit to another stable orbit. This results in a
discrete spectrum.
Wavelength of Hα line :
Hα line is formed when an
electron jumps from nf = 3 to ni = 2 orbit. It is the
Balmer series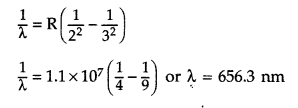
(b) Radius of nth orbit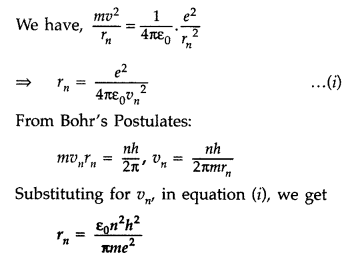
Question 10.
State Bohr’s postulate of the hydrogen atom successfully
explains the emission lines in the spectrum of the hydrogen atoms.
Use the
Rydberg formula to determine the wavelength of Ha line. [Given Rydberg constant
R = 1.03 × 107 m-1] (CBSE AI 2015)
Answer:
It states
that an electron might make a transition from one of its specified non¬radiating
orbits to another of lower energy. When it does so, a photon is emitted having
energy equal to the energy difference between the initial and final states. The
frequency of the emitted photon is then given by
hv = Ei – Ef where Ei and Ef are
the energies of the initial and final states
Using the formula
\(\frac{1}{λ}\) =
RH\(\left(\frac{1}{n_{f}^{2}}-\frac{1}{n_{i}^{2}}\right)\) we have
for Ha Line ni = 3 and nf = 2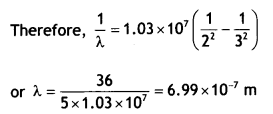
Question 11.
Using Bohr’s postulates derive the expression for the
frequency of radiation emitted when an electron In a hydrogen atom undergoes a
transition from a higher energy state (quantum number n-) to the towering state
(n,). When an electron in a hydrogen atom jumps from the energy state
ni =4 to n = 3, 2, 1, identify the spectral series to which the
emission lines belong. (CBSE Delhi 201 1C)
Answer:
According to Bohr’s
frequency condition, if an electron jumps from an energy Level E to
E1, then the frequency of the emitted radiation is given by
hv = E
– E1 …(1)
Let ni and nf be the corresponding orbits then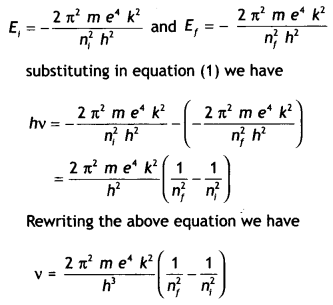
This gives the frequency of the emitted radiation.
When ni =4 and
nf = 3, Paschen series
When ni = 4 and nf =
2, Balmer series
When ni = 4 and nf = 1, Lyman
senes
Question 12.
Calculate the ratio of the frequencies of the radiation
emitted due to the transition of the electron In a hydrogen atom from Its (i)
second permitted energy level to the first level and (ii) highest permitted
energy level to the second permitted level. (CBSE Delhi 2018C)
Answer:
We
have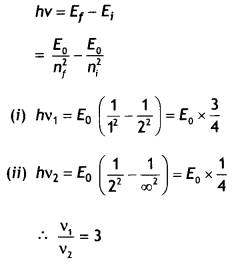
Question 13.
Monochromatic radiation of wavelength 975 A excites the hydrogen
atom from its ground state to a higher state. How many different spectral lines
are possible In the resulting spectrum? Which transition corresponds to the
longest wavelength amongst them? (CBSE Sample Paper 201819)
Answer:
The
energy corresponding to the given wavelength: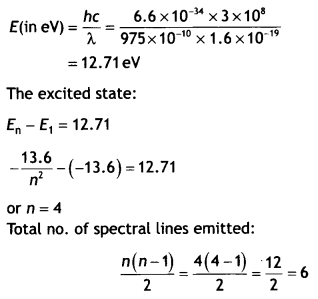
The longest wavelength Will correspond to the transition n = 4 to n = 3
Question 14.
(a) Using postulates of Bohr’s theory of hydrogen atom, show
that
(i) the radii of orbits increases as n², and
Answer:
Let us
consider a mechanical. model of the hydrogen atom as shown in the
figure.
This atom consists of a single electron with mass m and charge – e revolving
around a single proton of charge + e. As the electron revolves around the
nucleus the electrostatic force of attraction between the electron and the
proton provides the necessary centripetal force. Therefore we have,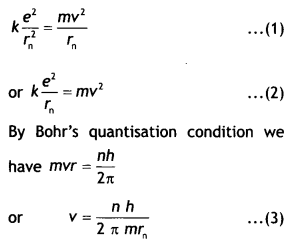
Substituting equation 3 in equation 2 we have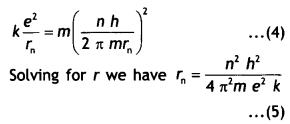
This gives the radius of the nth orbit of the hydrogen atom which
shows that E ∝ \(\frac{1}{n^{2}}\)
(ii) the total energy of the electron increases as 1/n², where n is the
principal quantum number of the atom.
Answer:
the total energy possessed
by an electron in the nth orbit of the hydrogen atom is given by
E = T + U
…(1)
i.e. the sum of its kinetic and electrostatic potential energies.
An electron of mass m moving around the nucleus with an orbital velocity v
has kinetic energy given by
K.E. = \(\frac{1}{2}\)mv² = \(\frac{1}{2}\frac{k
e^{2}}{r}\) ….(2)
Now the potential energy of the electron at a distance r from the nucleus is
given by
PE = potential due to the nucleus at a distance r × charge on the
electron
= V × – e …(3)
Now the potential at a distance r from the nucleus having a charge e is given
by
V = k\(\frac{e}{r}\) ….(4)
Substituting in equation 2 we have
P.E. = V × – e = – k
\(\frac{e^{2}}{r}\) …(5)
Substituting equations 2 and 5 in equation 1 we have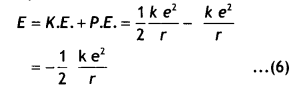
But the radius of the nth orbit is given by rn = \(\frac{n^{2} h^{2}}{4 \pi^{2} m e^{2} k}\)
Substituting in equation 6 we have
E = – \(\frac{2 \pi^{2} m e^{4}
k^{2}}{n^{2} h^{2}}\) …(7)
This gives the expression for the energy possessed by the eLectron in the nth orbit of the hydrogen atom which shows that E ∝ \(\frac{1}{n^{2}}\)
(b) Calculate the wavelength of H2 line In Balmer series of hydrogen atom,
given Rydberg constant R = 1.097 × 107 m-1. (CBSE AI
2011C)
Answer:
For H2 Line in Balmer series n1 = 2
and n2 = 3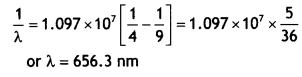
Question 15.
State Bohr’s quantization condition for defining stationary
orbits. How does de Brogue hypothesis explain the stationary orbits?
Find the
relation between the three wavelengths λ1 λ2 and λ3 from the energy level
diagram shown below. (CBSE Delhi 2016)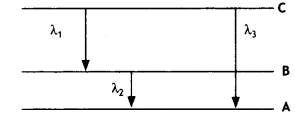
Answer:
It states that only those orbits are permitted in which the angular
momentum of the electron about the nucleus is an integral multiple of
\(\frac{h}{2π}\), where his Planck’s constant.
According to de Broglie, an electron of mass m moving with speed v would have
a wavelength λ given by
λ = h/mv.
Now according to Bohr’s postulate,
mvrn = \(\frac{n h}{2
\pi}\)
or
2πrn = \(\frac{nh}{mv}\)
But h / mv = A is the de BrogUe wavelength of the electron, therefore, the
above equation becomes 2πrn = nλ where 2πrn is the
circumference of the permitted orbit. If the wavelength of a wave does not close
upon itself, destructive interference takes place as the wave travels around the
loop and quickly dies out. Thus only waves that persist are those for which the
circumference of the circular orbit contains a whole number of
wavelengths.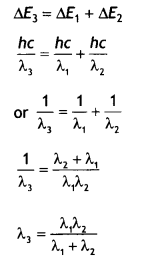
Numerical Problem :
Formulae for solving numerical problems
- Distance of closest approach ro = \(\frac{1}{4 \pi \varepsilon_{0}} \frac{2 z E^{2}}{E_{k}}\)
- Radius of the nth orbit of hydrogen atom rn = \(\frac{n^{2} h^{2}}{4 \pi^{2} m e^{2} k}\)
- Velocity of eLectron in the ntt orbit v = \(v=\frac{c}{137 n}\)
- Wavelength of radiation emitted when electron jumps form ni to nf \(\frac{1}{\lambda}=R_{\mathrm{H}}\left(\frac{1}{n_{\mathrm{f}}^{2}}-\frac{1}{n_{\mathrm{i}}^{2}}\right)\)
- Energy of electron in the nth orbit of hydrogen atom
E = – \(\frac{2 \pi^{2} m e^{4} k^{2}}{n^{2} h^{2}}\)
or
E = – \(\frac{13.6}{n^{2}}\) eV
Question 1.
A hydrogen atom in its excited state emits radiations of
wavelengths 1218 Å and 974.3 Å when it finally comes to the ground state.
Identify the energy levels from where transitions occur. Given Rydberg constant
R = 1.1 × 107 m-1. Also, specify the spectral series to
which these lines belong. (CBSE AI 2019)
Answer:
We know that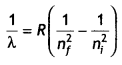
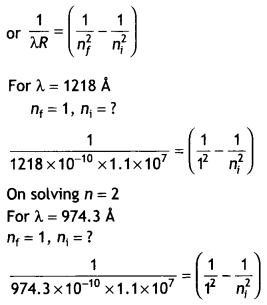
On solving n = 4
Lyman series.
Question 2.
Obtain the first Bohr’s radius and the ground state energy of
a muonic hydrogen atom, i.e. an atom where the electron is replaced by a
negatively charged muon (μ–) of mass about 207 me that orbits around
a proton. (Given for hydrogen atom, the radius of first orbit and ground state
energy are 0.53 × 10-10m and – 13.6 eV respectively) (CBSE AI
2019)
Answer:
In Bohr’s model of hydrogen atom the radius of
nth orbit is given by
rn = \(\frac{n^{2} h^{2}}{4
\pi^{2} e^{2} m_{e} k}\)
As n = 1
Therefore, we have 1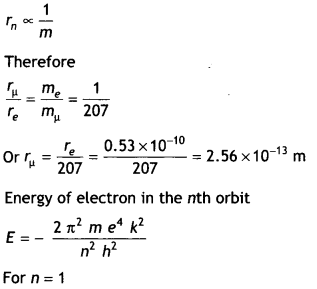
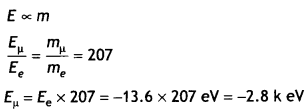
Question 3.
The electron in a given Bohr orbit has a total energy of – 1.5
eV. Calculate Its
(a) kinetic energy.
(b) potential energy.
(C) the
wavelength of radiation emitted, when this electron makes a transition to the
ground state.
(Given Energy in the ground state = – 13.6 eV and Rydberg’s
constant = 1.09 × 1o7 m-1) (CBSE Delhi 2011C)
Answer:
Total energy of the electron In a Bohr’s orbit is – 1.5 eV
We know that kinetic energy of the electron in any orbt is half of the
potential energy in magnitude and potential energy is negative
(a) Total
energy = kinetic energy + potential energy
– 1.5 = Ek –
2Ek
1.5 = Ek
(b) Ep = – 2 × 1.5 = – 3 eV
(c) Energy released when the transition of this electron takes place from
this orbit to the ground state
= – 1.5 – (- 13.6)
= 12.1 eV
= 12.1 ×
1.6 × 1019 = 1.936 × 10-18 J
Let be the wavelength of the Light emitted then,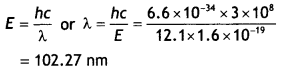
Question 4.
In a Geiger—Marsden experiment, calculate the distance of
closest approach to the nucleus of Z = 80, when an a-particle of 8 MeV energy
Impinges on it before It comes momentarily to rest and reverses its direction.
How will the distance of the closest approach be affected when the kinetic
energy of the a-particle is doubled? (CBSEAI 2012)
Answer:
Given Z = 80, E
= 8 MeV = 8 × 106 × 1.6 × 1019 J, ro =?
Using the expression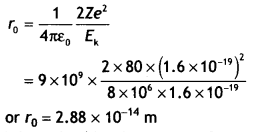
When the kinetic energy of a-particle has doubled the distance of the closest
approach becomes half its previous value, i.e. 1.44 × 1014 m
Question 5.
The ground state energy of the hydrogen atom is – 13.6 eV. If
an electron makes a transition from an energy level – 0.85 eV to – 3.4 eV,
calculate the wavelength of the spectral line emitted. To which series of
hydrogen spectrum does this wavelength belong? (CBSE AI 2012)
Answer:
Energy released = – 0.85 – (- 3.4) = 2.55 eV = 2.55 × 1.6 × 10-19
J
Using E = hc/λ we have
λ = \(\frac{h c}{E}=\frac{6.62 \times 10^{-34}
\times 3 \times 10^{8}}{2.55 \times 1.6 \times 10^{-19}}\) = 4.87 ×
10-7 m
It belongs to the Balmer series.
Question 6.
A hydrogen atom in the third excited state de-excites to the
first excited state. Obtain the expressions for the frequency of radiation
emitted in this process.
Also, determine the ratio of the wavelengths of the
emitted radiations when the atom de-excites from the third excited state to the
second excited state and from the third excited state to the first excited
state. (CBSEAI2019)
Answer:
We know that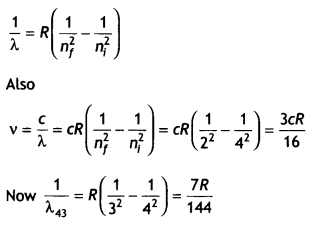
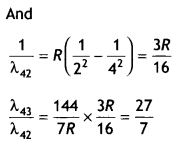
Question 7.
Obtain the expression for the ratio of the de Broglie wavelengths
associated with the electron orbiting in the second and third excited states of
the hydrogen atom. (CBSE Delhi 2019)
Answer:
We know that
2πr = nλ
….(i)
For the second excited state (n = 3)
r = 0.529(n)² A = 0.529(3)²
Putting in (i) we get 2π(0.529)(3)² = 3 λ2
For third excited state n = 4
r = 0.529 (4)²
Putting in (i) we get 2π
(0.529)(4)² = 4 λ3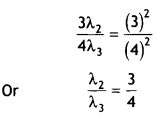
Question 8.
(a) The radius of the innermost electron orbit of a hydrogen
atom is 5.3 × 10-10 m. Calculate its radius in n = 3 orbit.
(b)
The total energy of an electron in the first excited state of the hydrogen atom
is 3.4 eV. Find out its (i) kinetic energy and (ii) potential energy in this
state. (CBSE Delhi 2014C)
Answer:
(a) The radius is given by
rn = \(\frac{n^{2} h^{2}}{4 \pi^{2} m e^{2} k}\) = n²ao
where n is the number of orbit, hence r3 = 5.3 × 10-10 × 32 = 4.77 × 10-9 m
(b) Total kinetic energy = + 3.4 eV
Total potential energy = – 6.8 eV
Question 9.
Given the ground state energy E0 = – 13.6 eV and Bohr radius
a0 = 0.53 Å. Find out how the de Broglie wavelength associated with the electron
orbiting in the ground state would change when it jumps into the first excited
state.
Answer:
The de-Broglie wavelength is given by 2πrn = nλ.
In
ground state, n = 1 and ro = 0.53 Å, therefore, λo = 2 ×
3.14 × 0.53 = 3.33 Å
In first excited state, n = 2 and r1 = 4 × 0.53 Å = 2.12 Å,
therefore,
r1 = (2 × 3.14 × 2.12)/2 = 6.66Å
Therefore, λ1 – λo = 6.66 – 3.33 = 3.33 Å.
In other
words, the de-Broglie wavelength becomes double.
Question 10.
When is the Hα line in the emission spectrum of
hydrogen atom obtained? Calculate the frequency of the photon emitted during
this transition. (CBSE AI 2016)
Answer:
Hα is obtained when ni
= 3 and nf = 2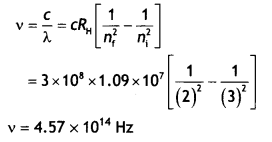
Question 11.
A 12.5 eV beam is used to excite a gaseous hydrogen atom at
room temperature. Determine the wavelength and the corresponding series of lines
emitted. (CBSE AI 2017)
Answer:
Given the energy of electron beam E = 12.5
eV
This will make the energy of the electrons 12.5 – 13.6 = – 1.1 eV.
Thus, the electrons of hydrogen will be raised to the third orbit.
Lines
emitted will be ni = 3, nf = 2 Balmer series.
The wavelength is given by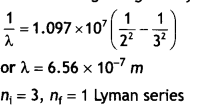
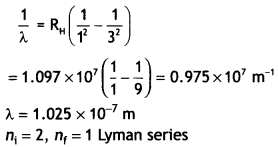
For first member ni = 2, therefore, the wavelength of the first
member of the Lyman series.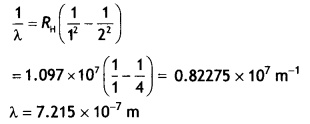
Question 12.
Calculate the longest wavelength of the photons emitted in
the Balmer series of the hydrogen spectrum. Which part of the e.m. spectrum does
it belong to? [Given Rydberg constant, R = 1.1 × 107 m-1
J] (CBSE Delhi 2017C)
Answer:
The longest wavelength is obtained when the
electron jumps from ni = 3 to nf = 2 in the Balmer series.
Therefore we have,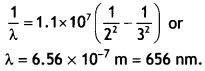
It lies in the visible region.
Question 13.
10 kg satellite circles earth once every 2 h in an orbit
having a radius of 8000 km. ‘ Assuming that Bohr’s angular momentum postulate
applies to satellites just as it does to an electron in the hydrogen atom, find
the quantum number of the orbit of the satellite. (NCERT Exemplar)
Answer:
From Bohr’s postulate, we have mvn rn =
nh/2π
Here m = 10 kg and rn = 8 × 106 m
We have the time period T of the circling satellite as 2 h.
That is T =
7200 s
Thus the velocity vn = 2πrn/T
The quantum number of the orbit of the satellite
N = (2πr)² × m/(T ×
h).
Substituting the values,
n = (2π × 8 × 106 )² × 10/(7200 × 6.64 ×
10-34)
= 5.3 × 1045
Note that the quantum number for the satellite motion is extremely large. In fact, for such large quantum numbers, the results of quantization conditions tend to those of classical physics.
Question 14.
Consider two different hydrogen atoms. The electron in each
atom is in an excited state. Is it possible for the electrons to have different
energies but the same orbital angular momentum according to the Bohr model?
Answer:
No, because according to the Bohr model, E = – 13.6 /n² eV and
electrons having different energies belong to different levels having different
values of n.
So, their angular momenta will be different, as nh/2π = mvr
Question 15.
Using the Bohr model, calculate the electric current created
by the electron when the H-atom is in the ground state. (NCEFU Exemplar)
Answer:
Let v be the velocity of the electron in the ground state. Let a0 be
the Bohr radius.
Now time is taken to complete one revolution
Question 16.
What is the minimum energy that must be given to an H atom in
the ground state so that it can emit a Hy line in the Balmer series?
If the angular momentum of the system is conserved, what would be the angular
momentum of such Hy photon? (NCERJ Exemplar)
Answer:
The
Hy in Balmer series corresponds to transition n = 5 to n = 2. So, the
electron in ground state n = 1 must first be put in state n = 5.
Hence energy required for the same.
Energy required = E1 –
E5 = 13.6 – 0.54 = 13.06 eV.
If angular momentum is conserved, angular momentum of photon = change in angular momentum of electron = L5 – L2 = 5h – 2h = 3h = 3 × 1.06 × 10-34 = 3.18 × 10-34 kg m² s-1