Chapter 12 Aldehydes, Ketones and Carboxylic Acids
Class 12th Chemistry Chapter Hots
1. Explain the preparation and properties of aldehydes and ketones by oxidation of alcohols.
Answer: Aldehydes and ketones are organic compounds containing a carbonyl group (C=O). The preparation of aldehydes and ketones through the oxidation of alcohols is a fundamental method.
Preparation of Aldehydes: Aldehydes are typically prepared by the oxidation of primary alcohols. Common oxidizing agents include pyridinium chlorochromate (PCC) or potassium dichromate (K₂Cr₂O₇). The reaction proceeds as:
An example is the oxidation of ethanol to acetaldehyde:
Preparation of Ketones: Ketones are prepared by the oxidation of secondary alcohols. In this case, a strong oxidizing agent like potassium dichromate (K₂Cr₂O₇) is often used. The reaction proceeds as:
For example, the oxidation of isopropanol (secondary alcohol) results in acetone:
Properties: Both aldehydes and ketones exhibit typical reactions of carbonyl compounds such as:
- Nucleophilic addition reactions: The carbonyl group is susceptible to nucleophilic attack.
- Reduction: Aldehydes are reduced to primary alcohols and ketones to secondary alcohols using reducing agents like NaBH₄ or LiAlH₄.
Example: Reduction of acetaldehyde (ethanal) with NaBH₄ gives ethanol, and reduction of acetone with NaBH₄ gives isopropyl alcohol.
2. Discuss the mechanism of nucleophilic addition to carbonyl compounds.
Answer: The nucleophilic addition mechanism to carbonyl compounds (such as aldehydes and ketones) involves several key steps:
-
Nucleophilic Attack: The nucleophile (a species with a lone pair of electrons, such as H⁻, CN⁻, or RMgX) attacks the electrophilic carbonyl carbon (C=O) because the oxygen is electron-rich and the carbon is electron-deficient.
-
Intermediate Formation: When the nucleophile attacks, the carbonyl double bond breaks, leading to the formation of a tetrahedral intermediate. This intermediate has a negative charge on the oxygen atom (since the oxygen is more electronegative).
-
Protonation: The negatively charged oxygen atom is protonated (usually by a solvent or another proton donor) to form the final addition product.
Example 1: In the reaction of acetaldehyde (CH₃CHO) with a cyanide ion (CN⁻), a cyanohydrin is formed:
Example 2: In the reaction of acetone (CH₃COCH₃) with a Grignard reagent (RMgX), a tertiary alcohol is formed after hydrolysis:
3. How does the structure of aldehydes and ketones influence their physical properties like boiling point, solubility, etc.?
Answer: Aldehydes and ketones exhibit distinct physical properties that are influenced by their structure:
-
Boiling Point: The boiling points of aldehydes and ketones are higher than those of alkanes, ethers, and hydrocarbons of similar molecular weight due to the presence of the polar carbonyl group (C=O). However, they are lower than those of alcohols because they cannot form hydrogen bonds between molecules, unlike alcohols, which have an -OH group.
-
Solubility: Small aldehydes and ketones (up to about 4 carbon atoms) are generally soluble in water due to their ability to form hydrogen bonds with water molecules through the oxygen atom. Larger aldehydes and ketones are less soluble in water due to the increasing hydrophobic nature of the carbon chain.
-
Intermolecular Forces: Aldehydes and ketones experience dipole-dipole interactions due to the polar nature of the C=O bond. The polarity of the carbonyl group influences their solubility in polar solvents and their ability to interact with other molecules.
Example: Acetone (CH₃COCH₃), with three carbon atoms, is miscible with water, whereas larger ketones like octanone are only slightly soluble.
4. What are the various methods of preparation of carboxylic acids?
Answer: Carboxylic acids can be synthesized by several methods, including:
-
Oxidation of Aldehydes: A common method is the oxidation of aldehydes. Aldehydes are oxidized to carboxylic acids using oxidizing agents like potassium dichromate (K₂Cr₂O₇) or alkaline KMnO₄.
Example: Acetaldehyde (CH₃CHO) can be oxidized to acetic acid (CH₃COOH).
-
Oxidation of Alcohols: Primary alcohols can be oxidized to carboxylic acids, with agents like K₂Cr₂O₇ or Na₂Cr₂O₇.
Example: Ethanol (CH₃CH₂OH) can be oxidized to acetic acid (CH₃COOH).
-
Hydrolysis of Nitriles: Nitriles (R-C≡N) can be hydrolyzed to carboxylic acids using aqueous acid or base.
Example: Hydrolysis of ethanitrile (CH₃CN) gives acetic acid (CH₃COOH).
-
Carbonation of Grignard Reagents: Grignard reagents (RMgX) can react with carbon dioxide (CO₂) to form carboxylic acids after hydrolysis.
Example: The reaction of methylmagnesium bromide (CH₃MgBr) with CO₂ forms acetic acid.
-
Kolbe’s Electrolysis: The Kolbe reaction involves the electrolysis of sodium salts of carboxylic acids to produce carboxylic acids.
Example: The electrolysis of sodium acetate (CH₃COONa) produces acetic acid.
5. Explain the mechanism of nucleophilic acyl substitution in the context of acyl chlorides and esters.
Answer: Nucleophilic acyl substitution is a common reaction mechanism for carboxylic acid derivatives such as acyl chlorides and esters. The process involves the replacement of the leaving group (Cl⁻ or OR) by a nucleophile (e.g., water, alcohol, amines).
Mechanism for Acyl Chlorides:
- Nucleophilic Attack: The nucleophile (such as water, alcohol, or amine) attacks the carbonyl carbon, leading to the formation of a tetrahedral intermediate.
- Leaving Group Departure: The leaving group (Cl⁻) departs from the intermediate.
- Protonation: The resulting product is protonated to yield the final product, such as a carboxylic acid (if water is used) or an ester (if alcohol is used).
Example: In the reaction of acetyl chloride (CH₃COCl) with water:
Mechanism for Esters: The esterification process involves the nucleophilic attack of alcohol on an acyl chloride or carboxylic acid. When ester reacts with a nucleophile, a similar substitution mechanism occurs.
6. What are the factors affecting the reactivity of carbonyl compounds?
Answer: The reactivity of carbonyl compounds (aldehydes, ketones, carboxylic acids) is influenced by several factors:
-
Electron-Withdrawing and Electron-Donating Groups: Electron-withdrawing groups (e.g., -NO₂, -Cl) attached to the carbonyl carbon make it more electrophilic and thus more reactive towards nucleophiles. On the other hand, electron-donating groups (e.g., -CH₃) reduce the electrophilicity of the carbonyl carbon.
-
Steric Effects: The size of substituents around the carbonyl group affects the reactivity. Aldehydes, having one hydrogen atom attached to the carbonyl carbon, are more reactive than ketones, which have two bulky alkyl groups.
-
Resonance Effects: The degree of conjugation (resonance) also affects the reactivity. Carboxylic acids and their derivatives are generally more reactive than aldehydes and ketones due to the additional resonance stabilization of the carbonyl group.
Example: Acetaldehyde (CH₃CHO) reacts more readily with nucleophiles than acetone (CH₃COCH₃) due to the absence of a bulky group in acetaldehyde.
7. How are carboxylic acids reduced to alcohols? Describe the process.
Answer: Carboxylic acids can be reduced to alcohols using a strong reducing agent like lithium aluminum hydride (LiAlH₄) or borane (BH₃). The reduction process involves the addition of hydride ions (H⁻) to the carbonyl carbon of the carboxyl group.
Mechanism:
- Hydride Attack: The hydride ion (H⁻) from LiAlH₄ attacks the carbonyl carbon in the carboxyl group, reducing the carbonyl bond.
- Intermediate Formation: The intermediate formed is an alkoxide ion (R-COO⁻).
- Protonation: The alkoxide is protonated during work-up with water to form the alcohol.
Example: Reduction of acetic acid (CH₃COOH) to ethanol (CH₃CH₂OH):
8. What are the physical and chemical properties of aldehydes and ketones? Explain with examples.
Answer: Aldehydes and ketones share similar physical and chemical properties due to the presence of the carbonyl group.
- Physical Properties:
- Boiling Point: Both have higher boiling points than alkanes due to dipole-dipole interactions between molecules but lower than alcohols.
- Solubility: Aldehydes and ketones are soluble in polar solvents like water, especially for small molecules (up to 4 carbon atoms).
- Chemical Properties:
- Reduction: Aldehydes reduce to primary alcohols, and ketones reduce to secondary alcohols.
- Nucleophilic Addition: As discussed in earlier questions, aldehydes and ketones undergo nucleophilic addition reactions.
- Oxidation: Aldehydes are easily oxidized to carboxylic acids, while ketones are resistant to oxidation.
Example: The oxidation of ethanol (CH₃CH₂OH) gives acetaldehyde (CH₃CHO), which can be further oxidized to acetic acid (CH₃COOH). Acetone (CH₃COCH₃) does not undergo oxidation under similar conditions.
9. What are the methods of preparation of aldehydes and ketones?
Answer: Aldehydes and ketones can be prepared by several methods:
-
From Alcohols:
- Aldehydes: Primary alcohols are oxidized to aldehydes using mild oxidizing agents like PCC (Pyridinium chlorochromate) or KMnO₄.
- Ketones: Secondary alcohols are oxidized to ketones using mild oxidizing agents like chromium compounds (e.g., Jones reagent, Na₂Cr₂O₇).
-
From Acyl Chlorides:
- Aldehydes: Acyl chlorides can be reduced to aldehydes using reducing agents like diborane (BH₃) or using Lindlar's catalyst in the case of the reduction of acid chlorides.
- Ketones: Acyl chlorides can also be reacted with organometallic compounds (Grignard reagents) to produce ketones.
-
From Alkenes (Hydroformylation Reaction):
- Aldehydes: Alkenes can undergo a hydroformylation reaction in the presence of a catalyst (like cobalt or rhodium) with carbon monoxide and hydrogen to form aldehydes.
- Ketones: Ketones can also be synthesized by reactions such as Friedel-Crafts acylation of alkyl groups with acyl halides.
Example:
- Preparation of acetaldehyde (CH₃CHO) from ethanol (CH₃CH₂OH) via oxidation with PCC:
- Preparation of acetone (CH₃COCH₃) from isopropyl alcohol (CH₃CH(OH)CH₃) via oxidation:
10. Explain the mechanism of nucleophilic addition reaction in aldehydes and ketones.
Answer: The nucleophilic addition mechanism in aldehydes and ketones involves the attack of a nucleophile on the electrophilic carbonyl carbon. The steps are as follows:
- Attack of Nucleophile: A nucleophile (like a hydride ion, alkoxide ion, cyanide ion, or Grignard reagent) attacks the carbonyl carbon, which is partially positive due to the electronegativity of oxygen.
- Formation of Tetrahedral Intermediate: The carbonyl carbon bonds to the nucleophile, forming a tetrahedral intermediate.
- Protonation: The intermediate is then protonated (if required), leading to the formation of a product such as an alcohol (in case of reduction) or a new compound (e.g., cyanohydrin in case of cyanide addition).
Example:
- Addition of cyanide ion (CN⁻) to acetaldehyde (CH₃CHO): This forms cyanohydrin, an important intermediate.
11. What is the difference between aldehydes and ketones?
Answer: The differences between aldehydes and ketones are as follows:
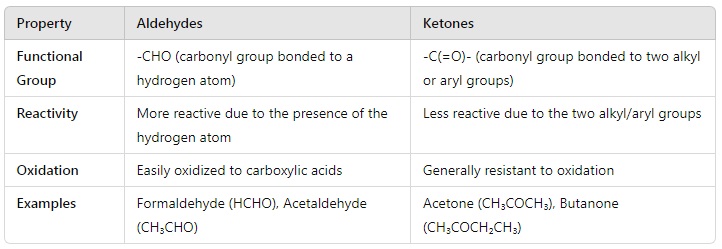
Example:
- Aldehyde: Formaldehyde (HCHO) is an aldehyde because it has a hydrogen attached to the carbonyl carbon.
- Ketone: Acetone (CH₃COCH₃) is a ketone because it has two methyl groups attached to the carbonyl carbon.
12. How do carboxylic acids differ from aldehydes and ketones in terms of their structure and reactivity?
Answer: Carboxylic acids differ from aldehydes and ketones both structurally and in terms of reactivity:
-
Structure: Carboxylic acids contain a -COOH (carboxyl) group, which consists of a carbonyl group (C=O) attached to a hydroxyl group (–OH), whereas aldehydes and ketones only contain a carbonyl group (C=O) without the hydroxyl group.
-
Reactivity:
- Carboxylic acids are more reactive than aldehydes and ketones due to the presence of the hydroxyl group, which can participate in hydrogen bonding and also undergo various reactions like esterification and decarboxylation.
- Aldehydes are more reactive than ketones because they have a hydrogen attached to the carbonyl carbon, which makes the carbonyl carbon more electrophilic.
Example:
- Carboxylic Acid: Acetic acid (CH₃COOH) can undergo esterification with alcohols to form esters, a reaction not possible for aldehydes and ketones in the same manner.
- Aldehyde: Acetaldehyde (CH₃CHO) can be easily oxidized to acetic acid (CH₃COOH), while ketones like acetone (CH₃COCH₃) are resistant to oxidation.
13. Explain the mechanism of esterification using carboxylic acids.
Answer: Esterification is the reaction between a carboxylic acid and an alcohol to form an ester. The process occurs in the presence of an acid catalyst, typically sulfuric acid.
Mechanism:
- Protonation of Carbonyl Oxygen: The carbonyl oxygen of the carboxylic acid is protonated by the acid catalyst, making the carbonyl carbon more electrophilic.
- Nucleophilic Attack: The alcohol (R-OH) attacks the electrophilic carbonyl carbon, forming a tetrahedral intermediate.
- Elimination of Water: The intermediate loses a water molecule, forming an ester (RCOOR').
- Regeneration of Catalyst: The acid catalyst is regenerated in the final step.
Example:
- Esterification of acetic acid with ethanol: This reaction produces ethyl acetate (CH₃COOC₂H₅) and water.
14. What are the uses of aldehydes, ketones, and carboxylic acids in daily life?
Answer: Aldehydes, ketones, and carboxylic acids have numerous applications in daily life:
- Aldehydes:
- Formaldehyde: Used as a disinfectant, preservative in biology labs, and in the production of resins and plastics.
- Acetaldehyde: Used in the manufacture of acetic acid and perfumes.
- Ketones:
- Acetone: A solvent used in nail polish remover, paint thinner, and as a cleaner.
- Methyl Ethyl Ketone (MEK): Used in the production of adhesives and paints.
- Carboxylic Acids:
- Acetic Acid: Found in vinegar, used as a preservative and in the food industry.
- Citric Acid: Used in the food and beverage industry, as a preservative and flavoring agent.
- Benzoic Acid: Used as a preservative in food products and in the manufacture of cosmetics and pharmaceuticals.
15. What are the methods of preparation of carboxylic acids?
Answer: Carboxylic acids can be prepared through several methods:
-
Oxidation of Aldehydes:
- Aldehydes are easily oxidized to carboxylic acids. For example, acetaldehyde (CH₃CHO) is oxidized to acetic acid (CH₃COOH) using an oxidizing agent like potassium permanganate (KMnO₄).
-
Oxidation of Alcohols:
- Primary alcohols can be oxidized to carboxylic acids. For example, ethanol (CH₃CH₂OH) can be oxidized to acetic acid (CH₃COOH) using an oxidizing agent like potassium dichromate (K₂Cr₂O₇).
-
Hydrolysis of Nitriles:
- Nitriles (R-C≡N) can be hydrolyzed under acidic or basic conditions to form carboxylic acids. For example, acetonitrile (CH₃CN) is hydrolyzed to acetic acid.
-
Kolbe's Electrolysis:
- Carboxylic acids can be prepared by electrolysis of sodium salts of carboxylic acids (e.g., sodium acetate) in the presence of dilute sodium hydroxide. This is known as Kolbe's electrolysis.
-
Benzene and Its Derivatives:
- Carboxylic acids can also be synthesized by the oxidation of alkyl benzenes (e.g., toluene) using strong oxidizing agents like KMnO₄.
16. How do aldehydes and ketones react with Grignard reagents?
Answer: Aldehydes and ketones react with Grignard reagents (RMgX) in a nucleophilic addition reaction. The mechanism is as follows:
- Nucleophilic Attack:
- The Grignard reagent (RMgX) acts as a nucleophile. The alkyl group (R) in the Grignard reagent attacks the electrophilic carbonyl carbon of the aldehyde or ketone.
- Formation of Tetrahedral Intermediate:
- This leads to the formation of a tetrahedral intermediate with the Grignard reagent bonded to the carbonyl carbon.
- Protonation:
- The intermediate is protonated upon work-up (addition of water) to give an alcohol.
Example:
- Reaction of acetaldehyde (CH₃CHO) with methyl magnesium bromide (CH₃MgBr): This forms 2-propanol (CH₃CH(OH)CH₃).
17. Explain the effect of oxidation on aldehydes and ketones.
Answer: Oxidation leads to different products when performed on aldehydes and ketones:
-
Aldehydes:
- Aldehydes are easily oxidized due to the presence of the hydrogen atom attached to the carbonyl carbon. When oxidized, they are converted to carboxylic acids.
- Example: Acetaldehyde (CH₃CHO) is oxidized to acetic acid (CH₃COOH).
- Strong oxidizing agents like potassium permanganate (KMnO₄) or potassium dichromate (K₂Cr₂O₇) can be used for oxidation.
-
Ketones:
- Ketones are generally more resistant to oxidation compared to aldehydes because they do not have a hydrogen atom attached to the carbonyl group. They require stronger oxidizing agents to be oxidized.
- Under extreme conditions, ketones may be oxidized to carboxylic acids, but typically only when the alkyl groups attached to the ketone are susceptible to cleavage.
18. What are the methods of detection of aldehydes and ketones?
Answer: Aldehydes and ketones can be detected using several tests:
-
Tollens' Test (Silver Mirror Test):
- Aldehydes give a silver mirror when treated with Tollens’ reagent (ammoniacal silver nitrate), but ketones do not react.
- Reaction:
-
Fehling's Test:
- Aldehydes reduce Fehling’s solution (a mixture of copper sulfate and alkali tartrate) to form a red precipitate of copper(I) oxide, while ketones do not react.
- Reaction:
-
Schiff's Test:
- Aldehydes react with Schiff’s reagent (fuchsine dye in sulfurous acid) to form a pink color, while ketones do not give a color change.
-
Iodoform Test:
- Both aldehydes and ketones that have a methyl group attached to the carbonyl group (CH₃CO-) can react with iodine in the presence of alkali to form a yellow precipitate of iodoform (CHI₃).
- Reaction:
19. Explain the mechanism of the reaction between aldehydes and Grignard reagents.
Answer: When an aldehyde reacts with a Grignard reagent (RMgX), it undergoes a nucleophilic addition reaction. The mechanism is as follows:
-
Attack of the Grignard Reagent:
- The Grignard reagent (RMgX) has a nucleophilic alkyl group (R). The nucleophilic carbon of the Grignard reagent attacks the electrophilic carbonyl carbon in the aldehyde.
-
Formation of Tetrahedral Intermediate:
- This attack leads to the formation of a tetrahedral intermediate, where the carbonyl oxygen gets a negative charge.
-
Protonation:
- After the intermediate is formed, the negatively charged oxygen is protonated (usually in the presence of water or dilute acid), leading to the formation of an alcohol.
Example:
- Reaction of acetaldehyde (CH₃CHO) with methylmagnesium bromide (CH₃MgBr): This results in the formation of 2-propanol.
20. What is the significance of the iodoform test for aldehydes and ketones?
Answer: The iodoform test is a chemical reaction used to identify aldehydes and ketones that have a methyl group attached to the carbonyl group. This test is specifically used to identify compounds with the structure CH₃CO-, such as acetone and methyl ketones.
Mechanism:
-
When an aldehyde or ketone with a methyl group (CH₃CO-) reacts with iodine (I₂) in the presence of an alkaline solution, the compound is oxidized, and a yellow precipitate of iodoform (CHI₃) is formed.
-
This reaction involves the halogenation of the methyl group attached to the carbonyl carbon, leading to the formation of iodoform.
Example:
- Acetone (CH₃COCH₃) reacts with iodine and alkali to form iodoform (CHI₃): The yellow precipitate of iodoform confirms the presence of the methyl ketone group.
21. What are the different methods of preparation of aldehydes and ketones?
Answer: Aldehydes and ketones can be prepared using various methods:
-
Oxidation of Alcohols:
- Aldehydes can be prepared by the partial oxidation of primary alcohols. For example, ethanol is oxidized to acetaldehyde.
- Ketones can be prepared by the oxidation of secondary alcohols. For example, isopropanol is oxidized to acetone.
-
Hydrolysis of Nitriles:
- Nitriles (R–CN) undergo hydrolysis to form carboxylic acids. However, in the case of nitriles like acetonitrile, the hydrolysis initially forms an amide and subsequently an aldehyde.
-
Reduction of Carboxylic Acids:
- Aldehydes can be prepared by reducing carboxylic acids with reducing agents like lithium aluminum hydride (LiAlH₄).
-
Ozonolysis of Olefins:
- Ketones are prepared by the ozonolysis of alkenes. The reaction involves the addition of ozone (O₃) to alkenes, resulting in the formation of aldehydes and ketones.
-
Friedel-Crafts Acylation:
- Ketones are prepared by the Friedel-Crafts acylation reaction, where an alkyl or aryl group is added to an acyl group (R–CO).
This forms an aromatic ketone.
22. Explain the acidity of carboxylic acids.
Answer: Carboxylic acids are weak acids, and their acidity can be explained based on the structure of the carboxyl group (–COOH). The acidity of carboxylic acids arises from the following factors:
-
Resonance Stabilization:
- In carboxylic acids, the proton (H⁺) is released from the hydroxyl group (–OH). The negative charge on the conjugate base (RCOO⁻) is delocalized due to resonance, where the negative charge is spread over both oxygen atoms.
The delocalization of the negative charge on the oxygen atoms stabilizes the conjugate base, making it easier for the acid to lose a proton.
-
Inductive Effect:
- The presence of electronegative substituents (like halogens) in the vicinity of the carboxyl group increases the acidity by stabilizing the conjugate base via the inductive effect. For example, chloroacetic acid (ClCH₂COOH) is more acidic than acetic acid (CH₃COOH) due to the electron-withdrawing effect of chlorine.
-
Comparison with Other Functional Groups:
- Carboxylic acids are more acidic than alcohols and phenols due to the greater resonance stabilization of the conjugate base in carboxylic acids.
23. How does the presence of electron-withdrawing and electron-donating groups affect the acidity of carboxylic acids?
Answer: The acidity of carboxylic acids is influenced by the nature of substituents attached to the benzene ring or the alkyl chain. The two key effects to consider are:
-
Electron-withdrawing Groups (EWGs):
- Substituents such as nitro (-NO₂), halogens (-Cl, -Br), and cyano (-CN) groups, which withdraw electron density from the carboxyl group through induction or resonance, increase the acidity of the carboxylic acid.
- Example: Nitrobenzoic acid (C₆H₄NO₂COOH) is more acidic than benzoic acid (C₆H₅COOH) because the -NO₂ group withdraws electron density from the carboxyl group, stabilizing the conjugate base.
-
Electron-donating Groups (EDGs):
- Groups such as methyl (-CH₃), hydroxyl (-OH), and alkoxy (-OCH₃) donate electron density to the carboxyl group, decreasing the acidity of the carboxylic acid by destabilizing the conjugate base.
- Example: Methylacetic acid (CH₃COOH) is less acidic than acetic acid (CH₃COOH) because the methyl group donates electron density to the carboxyl group, destabilizing the conjugate base.
24. Explain the mechanism of nucleophilic addition of cyanide to aldehydes and ketones.
Answer: The nucleophilic addition of cyanide to aldehydes and ketones involves the following steps:
- Nucleophilic Attack:
- The cyanide ion (CN⁻) acts as a nucleophile and attacks the electrophilic carbonyl carbon in aldehydes or ketones.
- The carbonyl carbon, being partially positive due to the electronegativity of oxygen, is susceptible to attack by the nucleophile.
- Formation of Tetrahedral Intermediate:
- This attack forms a tetrahedral intermediate with the cyanide ion attached to the carbonyl carbon. The oxygen of the carbonyl group now carries a negative charge.
- Protonation:
- The negatively charged oxygen of the intermediate is protonated by water (or acid during work-up), leading to the formation of a hydroxynitrile.
Example: The reaction of acetaldehyde with cyanide:
The product is hydroxyacetonitrile.
25. What is the role of carboxylic acids in the formation of esters?
Answer: Carboxylic acids react with alcohols to form esters in a reaction known as esterification, typically in the presence of a strong acid catalyst such as sulfuric acid (H₂SO₄). This is a nucleophilic substitution reaction.
Mechanism:
- Activation of the Carboxyl Group:
- The sulfuric acid protonates the carbonyl oxygen, making the carbonyl carbon more electrophilic and thus more susceptible to nucleophilic attack.
- Nucleophilic Attack by Alcohol:
- The alcohol (R’OH) attacks the carbonyl carbon of the carboxylic acid, forming a tetrahedral intermediate.
- Elimination of Water:
- The intermediate undergoes dehydration, eliminating water and forming the ester.
Example: The reaction of acetic acid (CH₃COOH) with ethanol (C₂H₅OH):
This results in the formation of ethyl acetate (CH₃COOC₂H₅).