Chapter 1 The Solid State
Class 12th Chemistry Chapter Hots
Here’s a detailed and long-format set of HOTS questions and answers for CBSE Class 12 Chemistry Chapter 1: The Solid State. Each answer is explained in depth, incorporating theoretical concepts, analytical thinking, and numerical applications.
1. Explain the various types of crystal systems and their characteristics. Derive the relationship between the unit cell parameters for cubic and tetragonal systems.
Answer:
Crystals are classified into seven crystal systems based on their unit cell
dimensions and interaxial angles. These systems include:
-
Cubic:
- Axial lengths:
- Axial angles:
-
Tetragonal:
- Axial lengths:
- Axial angles:
-
Orthorhombic:
- Axial lengths:
- Axial angles:
-
Hexagonal:
- Axial lengths:
- Axial angles:
-
Monoclinic:
- Axial lengths:
- Axial angles:
-
Triclinic:
- Axial lengths:
- Axial angles:
-
Rhombohedral:
- Axial lengths:
- Axial angles:
Relationship for Cubic System:
In the cubic system, the edge length is directly related to the atomic radius . For different arrangements:
- Simple Cubic (SC): .
- Body-Centered Cubic (BCC): .
- Face-Centered Cubic (FCC): .
Relationship for Tetragonal System:
In tetragonal systems, the volume of the unit cell is , where is the edge length of the square base, and is the height.
- Example: In a tetragonal crystal with and , .
2. Discuss the concept of voids in crystal structures. How are tetrahedral and octahedral voids formed, and why are they significant in ionic solids?
Answer:
Voids are empty spaces between atoms in a close-packed structure. In an ideal
close packing, atoms occupy about
of
the space, leaving
voids. Voids are categorized as:
-
Tetrahedral Voids:
Formed when a triangular base of atoms in one layer is capped by one atom from the adjacent layer.- Radius ratio ( ) for fitting: .
-
Octahedral Voids:
Formed by two interpenetrating triangular planes, one inverted relative to the other.- Radius ratio ( ) for fitting: .
Significance:
- Ionic solids: Smaller cations occupy tetrahedral voids, and larger cations occupy octahedral voids.
- Example: In NaCl, Cl ions form FCC lattice, and Na ions occupy octahedral voids.
Numerical Example:
Calculate the number of octahedral voids in FCC lattice of NaCl.
- In FCC, there are atoms per unit cell.
- Number of octahedral voids =
per
atom =
.
Thus, Na ions occupy octahedral voids.
3. Derive the expression for the density of a unit cell and calculate the density of iron given that it crystallizes in a BCC lattice with an edge length of and atomic mass of .
Answer:
The density
of a unit cell is given by:
Where:
- = Number of atoms per unit cell.
- = Molar mass of the element.
- = Edge length of the unit cell in cm.
- = Avogadro's number ( ).
For BCC lattice: .
Substituting values:
Calculation yields:
4. What are Schottky and Frenkel defects? Derive the expression for the number of Schottky defects in a solid at a given temperature.
Answer:
Schottky Defect:
Occurs when equal numbers of cations and anions are missing from the lattice.
- Results in a decrease in density.
- Common in ionic solids with high coordination numbers (e.g., NaCl).
Frenkel Defect:
Occurs when a smaller ion (usually a cation) is displaced from its lattice site
to an interstitial site.
- Does not affect density.
- Common in solids with low coordination numbers (e.g., ZnS).
Expression for Schottky Defects:
Let be the number of Schottky defects.
At equilibrium,
,
where:
- = Total number of lattice sites.
- = Enthalpy for defect formation.
- = Temperature in Kelvin.
- = Gas constant.
5. Explain band theory of solids. Why do conductors, insulators, and semiconductors have different electrical properties? Provide examples.
Answer:
Band Theory:
- In solids, atomic orbitals overlap to form energy bands.
- Two important bands:
- Valence Band: Occupied by valence electrons.
- Conduction Band: Higher energy band where electrons move freely.
Key Differences:
-
Conductors (e.g., Cu, Ag):
- Valence and conduction bands overlap.
- Electrons flow freely, enabling high conductivity.
-
Insulators (e.g., Diamond):
- Large energy gap ( ) between bands.
- Electrons cannot move, resulting in no conductivity.
-
Semiconductors (e.g., Si, Ge):
- Small energy gap ( ).
- Conductivity increases with temperature or doping.
Doping with phosphorus creates an n-type semiconductor, while doping with boron creates a p-type semiconductor.
6. Explain how X-ray diffraction is used to determine the structure of a crystal. Derive Bragg’s equation.
Answer:
X-ray diffraction helps in determining the arrangement of atoms in a crystal by
analyzing the pattern of X-rays scattered by the crystal.
Principle:
When X-rays strike the crystal planes, they are scattered. Constructive
interference occurs when the path difference between rays reflected from
successive planes is an integral multiple of the wavelength (
).
Derivation of Bragg’s Equation:
Consider parallel planes in a crystal separated by distance
:
- Path difference = , where is the angle of incidence.
- For constructive interference:
where is the order of reflection.
Application:
- Bragg’s equation is used to calculate interplanar spacing and analyze crystal structures.
Numerical Example:
If a beam of X-rays of wavelength is diffracted at an angle , calculate the interplanar spacing.
7. What is anisotropy? How does it arise in crystalline solids? Give examples of its importance in real-world applications.
Answer:
Anisotropy refers to the directional dependence of properties
in a crystalline solid. Properties like electrical conductivity, refractive
index, and thermal expansion vary with direction due to the ordered arrangement
of atoms.
Cause:
- Crystals have different atomic densities along different directions, causing varying interactions with external fields or forces.
Examples:
- Electrical Conductivity: Graphite is more conductive along its planes than perpendicular to them.
- Optics: Calcite shows double refraction due to its anisotropic nature.
Applications:
- Technology: Liquid crystal displays (LCDs) rely on anisotropic optical properties.
- Industry: Diamond cutting depends on its anisotropic hardness.
8. A compound forms a hexagonal close-packed (hcp) lattice. Calculate the number of atoms in a unit cell and the packing efficiency of the structure.
Answer:
In an hcp lattice:
-
Unit Cell Composition:
- Contribution from 12 corner atoms: .
- Contribution from 2 atoms on each face: .
- Total = atoms per unit cell.
-
Packing Efficiency:
Volume occupied by atoms in the unit cell:
Volume of the unit cell:
Efficiency:
9. Explain the differences between metallic, ionic, and covalent solids with examples. Why do covalent solids like diamond have high melting points?
Answer:
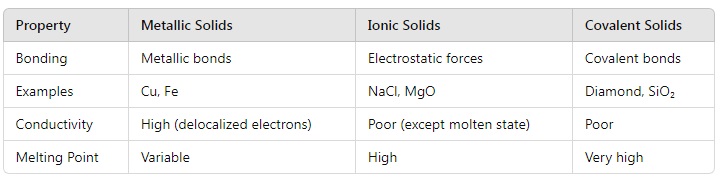
Explanation for Diamond:
- Diamond has a 3D network of strong covalent bonds. Breaking these bonds requires a lot of energy, leading to its exceptionally high melting point.
10. Differentiate between primitive and non-primitive unit cells. Discuss their significance in crystallography.
Answer:
Primitive Unit Cell:
- Atoms are present only at the corners.
- Simplest form of a unit cell.
- Example: Simple cubic lattice.
Non-Primitive Unit Cell:
- Atoms are present at corners and other positions (face, body, edge).
- Complex but accurately represents crystal symmetry.
- Examples: FCC, BCC.
Significance:
Non-primitive unit cells are crucial for understanding high packing efficiency and stability in crystals like NaCl (FCC).
11. Sodium crystallizes in a BCC lattice with an edge length of . Calculate its atomic radius and density.
Answer:
Atomic Radius for BCC:
Substitute :
Density Calculation:
For BCC:
.
Substitute
:
12. Explain the relationship between ionic radius and coordination number in ionic solids. Why does CsCl have a higher coordination number than NaCl?
Answer:
Relationship:
- Larger cations can accommodate more anions around them, increasing the coordination number.
- Radius ratio ( ) determines stability.
CsCl vs. NaCl:
- CsCl: Radius ratio is , allowing 8:8 coordination.
- NaCl: Radius ratio is , leading to 6:6 coordination.
13. Calculate the number of atoms per unit cell in an FCC lattice. Derive the packing efficiency.
Answer:
Number of Atoms:
- corner atoms: .
- face atoms: .
- Total: atoms.
Packing Efficiency:
14. Discuss the defects in solids with examples. Why do defects play an important role in materials science?
Answer:
Defects in solids are irregularities in the ideal arrangement of constituent
particles. These are categorized as:
(a) Point Defects:
- Vacancy Defect: Atoms missing from lattice points. Example: Alkali halides.
- Interstitial Defect: Extra atoms occupy interstitial positions. Example: Steel (C atoms in Fe).
- Substitutional Defect: Foreign atoms replace host atoms. Example: Brass (Zn replacing Cu).
(b) Line Defects:
- Irregularities along rows of atoms, such as dislocations.
Importance of Defects:
- Conductivity: Intrinsic defects increase carrier concentration in semiconductors.
- Strength: Line defects strengthen alloys (e.g., steel).
- Catalysis: Defect sites increase catalytic activity.
15. Calculate the density of a crystal given its edge length and molecular mass. Example: NaCl has , , FCC structure.
Answer:
Formula for density:
Substitute values:
- (FCC), , , :
16. Why does Frenkel defect occur in ionic solids? Give examples. How does it differ from Schottky defect?
Answer:
Frenkel Defect:
- Occurs when smaller ions (usually cations) leave their lattice positions and occupy interstitial sites.
- Common in solids with a large size difference between cations and anions, e.g., AgCl, ZnS.
Schottky Defect:
- Equal numbers of cations and anions are missing, reducing the density. Example: NaCl, KCl.
Key Differences:
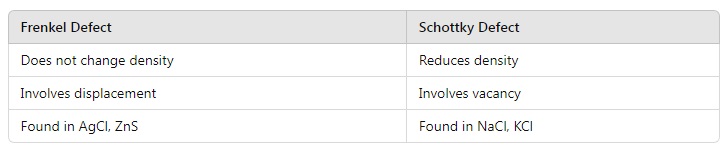
17. Explain the significance of radius ratio rules in determining the structure of ionic solids. Use the example of ZnS and NaCl.
Answer:
The radius ratio (
)
determines the coordination number (CN) and hence the structure of ionic solids.
Range of Ratios:
- Octahedral geometry, CN = 6 (e.g., NaCl).
- Tetrahedral geometry, CN = 4 (e.g., ZnS).
Examples:
- ZnS: Radius ratio , resulting in tetrahedral voids.
- NaCl: Radius ratio , leading to octahedral voids.
18. What is the importance of doping in semiconductors? How does n-type and p-type conductivity arise?
Answer:
Doping: Adding impurities to intrinsic semiconductors enhances
conductivity.
n-Type Semiconductor:
- Dopant: Pentavalent atoms (e.g., P, As) provide extra electrons, increasing conductivity.
- Majority carriers: Electrons.
p-Type Semiconductor:
- Dopant: Trivalent atoms (e.g., B, Al) create holes, which act as positive charge carriers.
- Majority carriers: Holes.
Applications:
Used in diodes, transistors, and solar cells.
19. Why does glass not exhibit a sharp melting point? How does it differ from crystalline solids?
Answer:
Glass is an amorphous solid, meaning its particles are randomly
arranged.
Properties of Amorphous Solids:
- Lack a definite melting point; they soften over a range of temperatures.
- Isotropic properties (uniform in all directions).
Difference from Crystalline Solids:
- Order: Crystalline solids have long-range order; amorphous solids have short-range order.
- Melting Point: Crystalline solids melt sharply, while amorphous solids soften gradually.
20. The conductivity of a metal decreases with an increase in temperature, whereas that of semiconductors increases. Explain.
Answer:
- Metals: As temperature increases, lattice vibrations increase, scattering electrons and reducing conductivity.
- Semiconductors: Higher temperatures provide energy to valence electrons to cross the band gap, increasing charge carriers and conductivity.
21. Discuss the relationship between lattice defects and electrical conductivity in ionic solids. Provide examples.
Answer:
Defects increase charge carriers:
- Schottky Defects: Increase ion mobility, enhancing conductivity. Example: NaCl at high temperatures.
- Frenkel Defects: Introduce interstitial ions, aiding conduction in AgCl.
22. Explain why graphite is soft and conducts electricity, but diamond is hard and an insulator.
Answer:
- Graphite:
- Soft due to weak van der Waals forces between layers.
- Conducts electricity due to delocalized -electrons.
- Diamond:
- Hard because of a 3D covalent bond network.
- Insulator as no free electrons are present.
23. Derive the expression for the volume of a unit cell in terms of the lattice parameter for a cubic system.
Answer:
For cubic lattices:
- Simple Cubic: .
- Body-Centered Cubic: Atoms touch along body diagonal ( ):
- Face-Centered Cubic: Atoms touch along face diagonal ( ):
24. Calculate the void space in a simple cubic unit cell.
Answer:
-
Volume of Atoms:
- 1 atom in the unit cell occupies volume:
-
Volume of Cube:
- Edge length , so volume:
-
Packing Efficiency:
-
Void Space:
25. A solid AB has a rock salt structure. If the edge length of the unit cell is and the density is , calculate the formula mass of AB.
Answer:
Using the formula:
where (for FCC), , and .
Substitute values:
Simplify to get , indicating the formula mass corresponds to NaCl.