Chapter 9 Biomolecules
Class 11th Biology NCERT Book Solution
NCERT Solutions For Class 11 Biology Biomolecules
NCRT TEXTBOOK QUESTIONS SOLVED
1. What are macromolecules?
Give examples.
Solution: Macromolecules
are large high molecular weight substances with complex molecular structure and
occur in colloidal state (being insoluble) in intracellular fluid. These are
formed by polymerization of large number of micromolecules. Polysaccharides,
proteins and nucleic acids are few examples.
2. Illustrate a glycosidic,
peptide, and a phospho- diester
bond.
Solution. (i) Glycosidic bond is
the type of chemical linkage between the monosaccharide units of disaccharides,
oligosaccharides and polysaccharides, which is formed by the removal of a
molecule of water.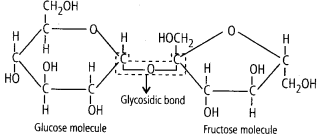
(ii)Peptide bonds are formed by the reaction between carboxyl
(- COOH) of one amino acid and amino (- NH2) group of other amino acid with the
elimination of water.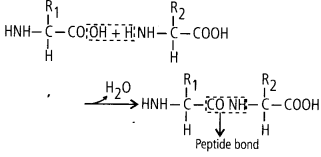
(iii) In a polynucleotide chain, adjacent nucleotides are
joined together by a bond called phosphodiester bond. This bond links a
phosphate group and sugar group of two adjacent nucleotides by means of an
oxygen bridge.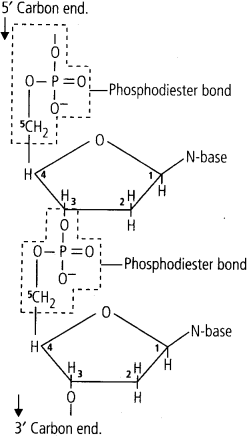
3. What is meant by tertiary
structure of proteins?
Solution: The
helical polypeptide molecule may fold on itself and assume a complex but
specific form-spherical, rod-like or any form in between these. These
geometrical shapes,are known as tertiary (3°) structure of protein molecules.
The coils and folds of the polypeptide molecules are so arranged as to hide the
non-polar amino acid chains inside and to expose the polar side chains. The
tertiary structure of a protein brings distant amino acid side chains nearer to
form active sites of enzymatic proteins. The tertiary structure is maintained by
weak bonds such as hydrogen, ionic, disulphide and hydrophilic – hydrophobic
bonds, formed between one part of a polypeptide and another. This structure is
easily disrupted by pH, temperature and chemicals stopping the function of
proteins.
4. Find and write down
structures ©f 10 interesting small molecular weight
biomolecules.
Solution: Interesfing
small molecular weight biomolecules are minerals (like sodium, potassium,
calcium, zinc, iodine etc), gases (like Oz, N2, C02, NH3) sugars – (ribose,
deoxyribose, glucose, fructose), lipids, amino acids, nucleotides (pyrimidines
& purine). Structures of 10 interesting small molecular weight biomolecules
are as follows: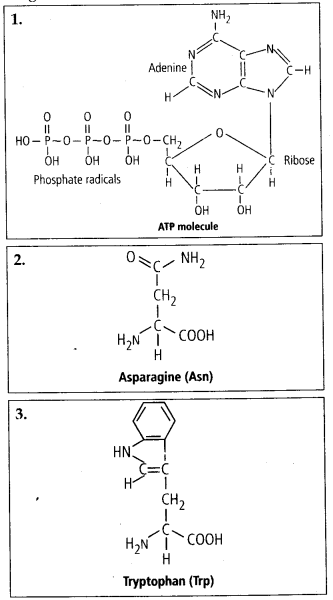
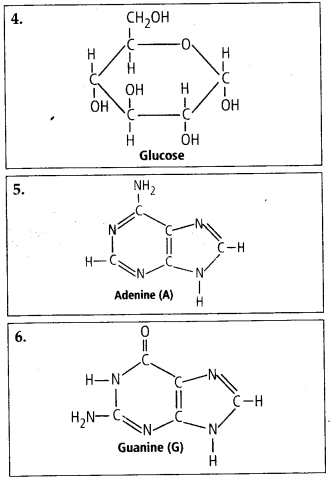
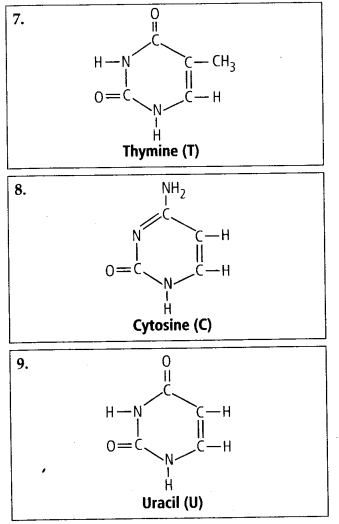
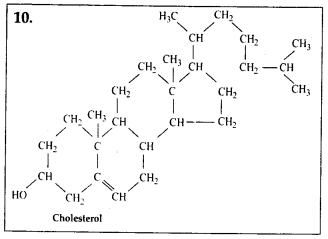
5. Proteins have primary
structure. If you are given a method to know which amino acid is at either of
two termini (ends) of a protein, can you connect this information to purity or
homogeneity of a protein?
Solution: There
are several methods provided by several scientists to find out the sequence of
amino acids. Frederick Sanger proposed Sanger’s reagent to know the amino acid
sequence in a polypeptide chain.
Sanger used 1-fluoro 2, 4 dinitrobenzene (FD
NB) to determine insulin structure. FDNB specifically binds with N-terminal
amino acid to form a dinitrophenyl (DNP) derivative of peptide. This DNP-
derivative peptide can be identified by chromatography. The identified sequence
of amino acids shows the homogeneity of a protein molecule.
6. Find out and make a list
of proteins used as therapeutic agents. Find other applications of
proteins.
Solution: Proteins used as
therapeutic agents are: thrombin, fibrinogen, enkephalins, antigens, antibodies,
streptokinase, protein tyrosine kinase, diastase, renin, insulin, oxytocin,
vasopressin etc. Proteins are also used in cosmetics, dairy industries, textile
industries, research techniques, biological buffers etc.
7. Explain the composition of
triglycerides. jSfflTriacylglycerols (triglycerides) are the esters of glycerol
with fatty acids.
Solution: They are
insoluble in water and non-polar in character and commonly known as neutral
fats. The neutral or depot fats are composed of carbon, hydrogen and oxygen like
carbohydrates but have far fewer oxygen atoms than carbon atoms unlike the
carbohydrates.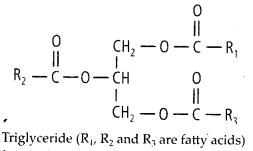
(i) Glycerol – A glycerol molecule has 3
carbons, each
bearing a hydroxyl (-OH) group. .
(ii) Fatty acids – A fatty acid molecule is
an unbranched chain of carbon atoms with each carbon atom (C) forming four bonds
to other atoms. It has a carboxyl group- COOH at one end and hydrogen atom (H)
bonded to all or most carbon atoms forming a hydrogen chain. The carbon-
hydrogen bonds are non-polar. Therefore, the hydrocarbon chain does not dissolve
in water. Because the carboxyl group contains the polar C = O and OFI groups. It
tends to dissolve in water even though the rest of fatty acid molecule will not.
Triacylglycerols of plants, in general, have higher content of unsaturated fatty
acids as compared to that of animals.
8. Can you describe what
happens when milk is converted into curd or yoghurt, from your understanding of
proteins.
Solution: Milk is converted
into curd or yoghurt due to denaturation of proteins. In denaturation,
disruption of bonds that maintains secondary and tertiary structure leads to the
conversion of globular proteins into fibrous proteins. This involves a change in
physical, chemical and biological properties of protein molecules.
9. Can you attempt building
models of biomolecules using commercially available atomic models (Ball and
stick models).
Solution: Yes, models of
biomolecules can be prepared using commercially available atomic models.
Ball
and stick models and space filling models are 3D or spatial molecular models
which serve to display the structure of chemical products and substances or
biomolecules. With ball and stick models, the centers of the atoms are connected
by straight lines which represent the covalent bonds. Double and triple bonds
are often represented by springs which form curved connections between the
balls. The bond angles and bond lengths reflect the actual relationships, while
the space occupied by the atoms is either not represented at all or only denoted
essentially by the relative sizes of the spheres.
10. Attempt titrating an
amino acid against a weak base and discover the number of dissociating
(ionizable) functional groups in the amino
acid.
Solution: The existence of
different ionic forms of amino acids can be easily understood by the titration
curves. The number of dissociating functional group is one in case of neutral
and basic amino acids and two in case of acidic amino acids.
11. Draw the structure of the
amino acid, alanine.
Solution: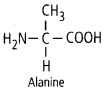
12. What are gums made of ?
Is fevicol different ?
Solution: Gums
are hetero-polysaccharides (poly-mers) of large number of different
monosac-charide units. Yes, fevicol is a different kind of polymer. It is a
synthetic sticky substance called resin which is manufactured by esteri-fication
of organic compounds.
13. Find out a qualitative
test for proteins, fats and oils, amino acids and test, any fruit juice, saliva,
and urine for them.
Solution: Biuret
test for protein : The biuret test is a chemical test used for determining the
presence of peptide bonds. In a positive test, a copper II ion (Cu2+ ion) is
reduced to copper I (Cu+) which forms a complex with the nitrogen and carbon of
peptide bonds in an alkaline solution. A violet colour indicates the presence of
proteins.
Ninhydrin test for amino acid: Ninhydrin (2,2 Dihydroxy
indane-l,3-dione) is a chemical used to detect ammonia or primary and secondary
amines. When reacting with these free amines, a deep blue or purple colour known
as Ruhemann’s purple is evolved. Amino acid analysis of proteins is also done by
ninhydrine. Most of the amino acids (including a-amino acids) are hydrolysed and
reacted with ninhydrin except proline (a secondary amine). Amino acid containing
a free amino group and a free carboxylic acid group reacts together with
ninhydrin to produce coloured product. When the amino group is secondary, the
condensation product is yellow.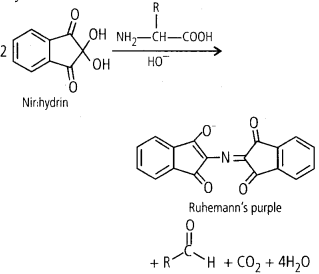
Solubility test for fats and oils : A positive solubility
test for fats is that the fat dissolves in lighter fluid and not in water. In
this test, 5 drops of fat or oil are added in two test tubes containing 10 drops
of lighter fluid and 10 drops cold water respectively.
Fruit juice contains
sugar so it cannot be tested by the above-mentioned tests. Saliva contains
proteins, mineral salts, amylase etc., so it can be tested for protein and amino
acids. Urine contains proteins, so it can be tested for it.
14. Find out how much
cellulose is made by all the plants in the
biosphere.
Solution: About 100 billion
tonnes of cellulose is prepared per year by the plants of the world.
15. Describe the important
properties of enzymes.
Solution: The
important properties of enzymes are as follows:
(i) The enzymes are generally
proteins which are high molecular weight complex globular proteins. They can
associate with non-protein substance for their activity.
(ii) The enzymes do
not start a chemical reaction but only accelerate it. They combine temporarily
with the substrate molecules and are not consumed or changed permanently in the
reaction which they catalyse.
(iii) The enzyme controlled reactions are
reversible.
(iv) The enzymes are specific in action. An enzyme catalyses only
a particular kind of reaction or acts on a particular substrate only.
(v) The
enzymes are thermolabile i.e., heat sensitive and can function best at an
optimum temperature. Similarly, enzymes show maximum activity at optimum pH.
(vi) The enzymes are inactivated by poisons and radiation.