CBSE Sample Papers for Class 10 Science Set-9
Class 10thCBSE Sample Papers for Class 10 Science Set-9
CBSE Sample Papers for Class 10 Science Set 9 with Solutions
Time: 3 Hours
Maximum Marks: 80
Instructions
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
- Section A consists of 20 objective-type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
- Section D consists of 3 Long Answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 4 marks each with sub-parts.
Section A
Select and write the most appropriate option out of the four options given for each of the questions 1-20.
Question 1.
When NaOH and HCl are mixed in equal molar quantities, the
result is
(a) the formation of salt + H2O
(b) the formation of
salt + H2 (g)
(c) the formation of salt + O2 (g)
(d)
the formation of salt + N2
Answer:
(a) the formation of salt +
H2O
When NaOH and HCl are mixed in equal molar quantities, the
acid-base reaction takes place and we get salt (NaCl) and water.
NaOH + HCl →
NaCl (Salt) + H2O
Question 2.
A light ray enters from medium A to medium B as shown in the
figure. The refractive index of medium B relative to A will be: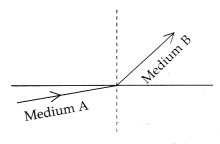
(a) greater than unity
(b) less than unity
(c) equal to
unity
(d) zero
Answer:
(a) greater than unity
Explanation: As the
light rays travel from medium A to medium B, then they bend towards the normal
which means that medium B has a higher refractive index and less speed of light
concerning medium A., So, the refractive index of medium B w.r.t. medium A will
be greater than unity.
Question 3.
Four metals Zn, Fe, Cu, and Al are taken and added to the
following solutions one by one. The results obtained are tabulated as given
below. Based on the data given which of the following elements is most
reactive?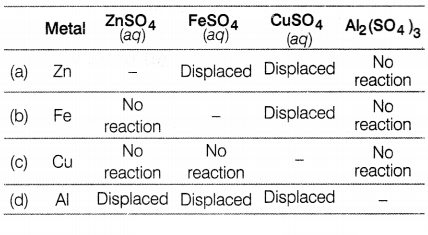
Answer:
From the results given in the table, Al is the most reactive among others
because it shows displacement reactions in three solutions, Zn shows,
displacement reactions in two solutions and iron shows displacement in one
solution. The least reactive metal is copper as it does not react with any
solution.
Question 4.
In which of the following aspects does multiple fission differ
from binary fission?
(i) Number of offspring produced.
(ii) Level of
genetic variation in offspring.
(iii) Number of parents involved.
(iv)
Multiple fission occurs in Plasmodium, whereas binary fission occurs in
Leishmania.
(a) Only (i) is correct
(b) Both (i) and (iv) are correct
(c) (iii) is correct
(d) (ii) is correct
Answer:
(b) Both (i) and (iv)
are correct
Explanation: Multiple fission produces many offspring whereas
binary fission produces only two. Off-spring produced through multiple fission
as well as binary fission are genetically identical to each other and to their
parents. Both multiple fission and binary fission require only one parent.
Plasmodium, the protozoan that causes malaria reproduces through multiple
fission. Leishmania causes Kala-azar and it reproduces through binary
fission.
Question 5.
Which of the following is not an heteroatom
CH2—O—CH2—CH2(Br) are
(a) oxygen
(b)
carbon
(c) hydrogen
(d) bromine
Answer:
(b) carbon
Oxygen (O).
chlorine (Cl) and bromine (Br) are heteroatoms. Please remember that apart from
C and H atoms, all other atoms present in an organic compound are hetero
atoms.
Question 6.
If a person has five resistors, each of value \(\frac{1}{5}\)
Ω, then the maximum resistance he can obtain by connecting them is:
(a) 1
Ω
(b) 5 Ω
(c) 10 Ω
(d) 25 Ω
Answer:
(a) 1 Ω
Explanation:
Resistance of one resistor = \(\frac{1}{5}\) Ω
Number of resistors = 5
Maximum resistances can be obtained by combining the resistors in a series:
Rs = R1 + R2 + R3 + R4 +
R5
Hence, a person on combining five resistors in a series gets
resistance 1 Ω.
Question 7.
Carbon forms four covalent bonds by sharing its four valence
electrons with four univalent atoms, e.g. hydrogen. After the formation of four
bonds, carbon attains the electronic configuration of
(a) helium
(b)
neon
(c) argon
(d) krypton
Answer:
(b) neon
The compound formed
is methane (CH4). In this, the carbon atom has a complete octet and
configuration of neon which is a noble gas element.
Question 8.
A plant is grown in a sealed container with a controlled
environment containing carbon dioxide, water, and sunlight. After some time, the
plant starts to show growth and produces oxygen. Which of the following
statements is most likely true?
(a) The plant is undergoing photosynthesis, a
form of autotrophic nutrition.
(b) The plant is obtaining nutrients from the
surrounding soil, a form of heterotrophic nutrition.
(c) The plant is
undergoing cellular respiration, a form of heterotrophic nutrition.
(d) The
plant is absorbing nutrients directly from the air, a form of autotrophic
nutrition.
Answer:
(a) The plant is undergoing photosynthesis, a form of
autotrophic nutrition.
Explanation: Autotrophic nutrition is the process by
which organisms produce their food using simple inorganic substances, such as
carbon dioxide, water, and sunlight, to synthesize organic compounds, like
glucose. In the given scenario, the plant is grown in a sealed container with
carbon dioxide, water, and sunlight, and it produces oxygen. This is a classic
indication of photosynthesis, j where plants use sunlight energy to convert
carbon dioxide and water into glucose and release oxygen as a byproduct.
Question 9.
Consider the following diagram that represents the
reproductive system in the human male.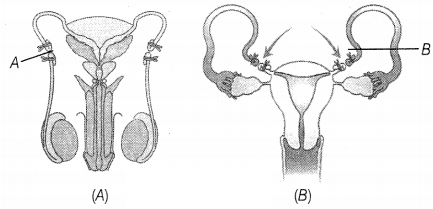
The blockages shown at A and B would most likely interfere with the ability
to
(a) transport gametes
(b) produce mature gametes
(c) eliminate waste
products through the urethra
(d) express secondary sex characters
Answer:
(a) transport gametes
The blockages shown at A and B would likely
interfere with the ability to transport gametes. Figure A shows the vasectomy in
which vas deferens are removed or tied up through a small incision on the
scrotum. Figure (B) shows tubectomy, in which a small part of the Fallopian tube
is removed or tied up through a small incision in the abdomen or through the
vagina.
Question 10.
The equivalent resistance of a series combination of two
resistances is X ohm. If the resistances are of 10 Ω and 40 Ω respectively, the
value of X will be:
(a) 10 Ω
(b) 20 Ω
(c) 50 Ω
(d) 40 Ω
Answer:
(c) 50 Ω
Explanation:
We know that
Total Resistance
R =
R1 + R2
= 10 + 40
= 50 Ω
Hence, the value of X is
50 Ω.
Question 11.
Which one of the following sets of options correctly depicts
reproduction in Amoeba and yeast, respectively?
(a) Budding and binary
fission
(b) Binary fission and budding
(c) Multiple fission and binary
fission
(d) Fragmentation and grafting
Answer:
(b) Binary fission and
budding
Binary fission in Amoeba and budding in yeast.
Question 12.
If we place the magnetic compass near the north pole of the
magnet, which pole of the needle will point towards it?
(a) North pole
(b) South pole
(c) Keep deflecting
(d) None of these
Answer:
(b) South pole
Explanation: As like poles
repel each other and unlike poles attract each other. Therefore when the North
pole of a bar magnet is brought near the compass, it gets deflected in the south
direction.
Question 13.
In circuit
(a) ammeter and voltmeter both are connected in
series.
(b) ammeter is connected in parallel and the voltmeter in series.
(c) ammeter is connected in series and voltmeter in parallel.
(d) ammeter and
voltmeter both are connected in parallel.
Answer:
(c) ammeter is connected
in series and voltmeter in parallel.
In any circuit, the ammeter should be
connected in series and the voltmeter should be connected in parallel.
Question 14.
A patient was diagnosed with a condition that resulted in the
obstruction of the bile duct. As a result, the patient experienced symptoms such
as jaundice and fatty stools. Which of the following is the primary function of
bile juice that is impaired in the patient with a blocked bile duct?
(a)
Emulsification of fats
(b) Neutralization of stomach acid
(c) Activation
of digestive enzymes
(d) Absorption of water and electrolytes
Answer:
(a) Emulsification of fats
Explanation: The primary function of bile juice is
the emulsification of fats. Bile is produced by the liver and stored in the
gallbladder. It is released into the small intestine to aid in the digestion and
absorption of dietary fats.
Bile contains bile salts, which act as emulsifiers. Emulsification is the process of breaking down large fat globules into smaller droplets.
In the case of a blocked bile duct,, the patient experiences symptoms such as jaundice (due to the accumulation of bilirubin, a bile pigment) and fatty stools (due to the malabsorption of dietary fats).
Question 15.
At the time of puberty, both boys and girls show lots of
changes in appearance. Select the hormone responsible for these changes in
boys.
(a) Oestrogen
(b) Adrenaline
(c) Testosterone
(d)
Thyroxine
Answer:
(c) Testosterone
Testosterone is responsible for
changes in boys during puberty.
Question 16.
The opening and closing of stomatal pores depends upon :
(a) Oxygen
(b) Water in guard cells
(c) Concentration of carbon dioxide in
stomata
(d) Temperature
Answer:
(b) Water in guard cells
Explanation: The entry of water into guard cells aids in the opening of guard
cells, the guard cell I becomes turgid because of this. Water going out from
guard cells aids in the closing of guard cells, because of this the guard cells
become flaccid.
Direction (Q. Nos. 17-20) consists of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both
A and R are true, but R is not the correct explanation of A.
(c) A is true,
but R is false.
(d) A is false, but R is true.
Question 17.
Assertion (A): Tungsten metal is selected for making
filaments of incandescent lamps.
Reason (R): Tungsten has a high melting
point.
Answer:
(a) Both A and R are true and R is the correct explanation
of A.
Tungsten has a high melting point. Therefore, it is used for making
filament of bulbs.
Question 18.
Assertion: Silver articles become black after sometime when
exposed to sunlight.
Reason: It is because silver reacts with carbonates
present in the air.
Answer:
(c) A is true but R is false
Explanation:
Silver reacts with sulphur present in the air and forms a layer of silver
sulphide, therefore, silver articles get tarnished or becomes black after
sometime when exposed to sunlight.
Question 19.
Assertion (A): In woody plants, gaseous exchange occurs
through lenticels.
Reason (R): Lenticels are specialized cells found along
with stomata on the stem of woody plants.
Answer:
(c) (A) is true, but (R)
is false.
In woody plants, gaseous exchange occurs through the small pores
found on stems called lenticels. Stomata on the stem aid in gaseous exchange, in
herbaceous plants.
Question 20.
Assertion: Electric current flowing through a metallic wire
is directly proportional to the potential difference across its ends.
Reason:
Ohm’s law expression V = IR, where R (resistance) of the wire is always
varying.
Answer:
(c) A is true but R is false
Explanation: Ohm’s law
states that the electric current flowing through a metallic wire is directly
proportional to the potential difference across its two ends. The expression is
written as :
V = IR
Here, R (resistance of the wire) is a constant value
then only the statement will be valid.
V ∝ I only if \(\frac{V}{I}\) =
constant
Section B
Questions No. 21 to 26 are Very Short Answer Questions.
Question 21.
Plaster of Paris is used to make sculptures and metal casting
is used as decorative material in buildings. It should be generally stored in
moisture-proof containers. Explain, why support your response with the help of a
chemical equation.
Answer:
Plaster of Paris (POP) is chemically calcium
sulphate hemihydrate (CaSO4 . \(\frac{1}{2}\)H2O). when it
comes in contact with water it sets into a hard solid mass, called
gypsum.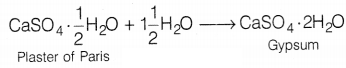
To prevent this, POP must be stored in moisture-proof containers. (2)
Question 22.
What is the importance of photosynthesis in the life of the
following :
(i) Green plants
(ii) Non-green plants
(iii) Animals
Answer:
(i) Green plants can build up complex energy-rich molecules of
carbohydrates which are further used for different metabolic activities of
cells.
(ii) non-green plants such as saprophytes and parasites use the food prepared by green plants during photosynthesis as a source of their own nutrition.
(iii) Animals eat green plants or eat animals that feed on green plants.
Question 23.
In the food chain given below, which organisms will be the
least in numbers?
Algae – Protozoan – Small fish – Large fish
Or
Why
are crop fields known as artificial ecosystems?
Answer:
In the given food
chain, i.e,
Algae → Protozoans → Small fish → Large fish
The large fish
(tertiary consumer/top carnivore) will have the least number of organisms. In
any food chain, there are generally a greater number of organisms at the lower
trophic levels. Thus, the producers have the greatest numbers and top carnivores
have the least numbers. (2)
Or
Artificial ecosystems are those ecosystems
that are modified and managed by human beings. Crop fields are man-made. Here
plants do not grow naturally rather most of the plants are grown by humans
according to the season, type of soil, etc. Crop fields are not like wild forest
area, which is left to the care of nature and can sustain themselves. In crop
fields, the land is managed, the soil is prepared for sowing seeds, then
irrigated, and further progress is also kept under observation to get a good
yield. This is why crop fields are known as artificial ecosystems. (2)
Question 24.
What is a parasitic mode of nutrition? Give examples of both
plants and animals that are parasites.
Answer:
There are different
strategies adopted by the organism for nutrition depending on how the food is
available. Some organisms break down food outside the body and then absorb it.
Others take in food into the body and then digest it. Some organisms get their
nutrition from plants and animals without killing them. These organisms are
called parasites and the organism from which they derive their food is called
host. Some of the parasites are lice, ticks, mites, leeches, and tapeworms among
animals and orchids and Cuscuta among plants.
Question 25.
The voltage-current (V-I) graph of a metallic circuit at two
different temperatures T1 and T2 is shown in the figure.
Which of the two temperatures is higher and why?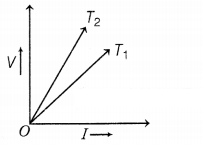
Or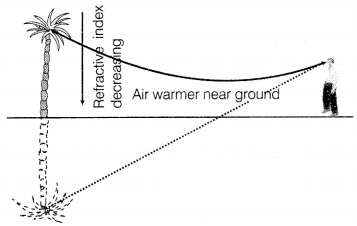
Explain the phenomenon and its cause in the above diagram. What will happen when
light travels from hot air to cold air?
Answer:
Resistance is equal to the
slope of the V-I graph. Here, slope of graph for temperature T2 is
higher, so resistance for temperature T2 is higher, As R ∝ T, T2 >
T1 (2)
Or
The phenomenon observed in the given figure is a
mirage. A mirage is formed due to the bending of light because of the
temperature difference between different layers of air. Due to this the
refractive index changes with height. When light travels from hot air (rarer) to
cold air (denser), then it bends towards the normal. (2)
Question 26.
While diluting an acid, why is it recommended that the acid
should be added to water and not water to the acid?
Answer:
The process of
dissolving an acid or a base in water is highly exothermic. The acid must always
be added slowly to water with constant stirring. If water is added to a
concentrated acid, the heat generated may cause the mixture to splash out and
cause burns. The glass container may also break due to excessive local heating.
Hence, it is recommended that the acid should be added to water and not water to
the acid.
Section C
Questions No. 27 to 33 are Short Answer Questions.
Question 27.
What is a homologous series of substances? Explain, why
carbon forms compounds mainly by covalent bonds.
Or
Name the type of
carbon compounds that can be hydrogenated. With the help of a suitable example
explain the process of hydrogenation.
Answer:
A series of similarly
constituted compounds in which the members present have the same functional
group and similar chemical properties and any two successive members in a
particular series differ in their molecular formula by (—CH2) unit is
called a homologous series, e.g. CH4, C2H8,
C3H8 are the members of alkane family. (2)
Carbon has 4
electrons in its outermost shell and needs to gain or lose 4 electrons to attain
a noble gas configuration. Losing or gaining 4 electrons is not possible due to
energy considerations, hence it shares electrons to form covalent bonds. (1)
Or
Only unsaturated hydrocarbons, i.e. alkenes and alkynes can be
hydrogenated.
e.g. In the presence of a catalyst Ni/Pd, ethyne is
hydrogenated into ethane.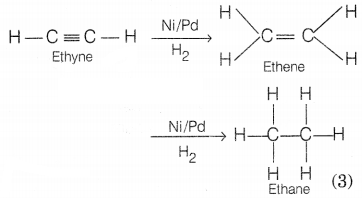
Question 28.
What is a thermal decomposition reaction? Give examples. A
student heats white lead nitrate [Pb(NO3)2] powder taken
in a test tube over the flame. Upon heating, the powder colour changes to yellow
along with the emission of some brown fumes. What is the expected product of
this decomposition reaction?
Answer:
Thermal decomposition is a
decomposition reaction carried out by heating. The decomposition of calcium
carbonate into calcium oxide and carbon dioxide on heating is an example of
thermal decomposition. The calcium oxide formed is also called lime or
quicklime.![]()
The expected product of the decomposition reaction of white
lead nitrate [Pb(NO3)2] is lead(II) oxide (PbO), yellow in
colour, along with the emission of nitrogen dioxide (NO2) gas, which
appears as brown fumes.
Here in the presence of heat decomposition is taking
place, so, it is known as thermal decomposition.
2Pb(NO3)2 → 2PbO + 4NO2 + O2
Question 29.
Mention the pathway of urine starting from the organ of its
formation. Name four substances that are reabsorbed from the initial filtrate in
the tubular part of the nephron.
Answer:
The pathway of urine starting
from the organ of its formation is as follows:
Kidneys → Ureters → Urinary
bladder → Urethra
- Kidney: It contains millions of complex nephrons and filters about 170 to 200 liters of blood. It produces 1-1.8 liters of urine daily.
- Ureters: These carry urine from the kidneys to the urinary bladder.
- Urinary bladder: This is a muscular sac-like structure where urine is stored until released.
- Urethra: This is a short muscular tube that carries urine from the urinary bladder to the outside of the body. (2)
The four substances reabsorbed from the initial filtrate are
- Amino acid
- Glucose
- Salts
- Major amount of water (1)
Question 30.
Write the molecular formula of the following compound and
draw their electron dot structure,
(i) Ethane
(ii) Ethene
(iii)
Ethyne
Answer:
(i) Ethane Molecular formula:
C2H6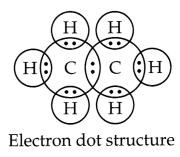
(ii) Ethene: Molecular formula:
C2H4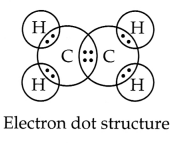
(iii) Ethyne: Molecular formula
C2H2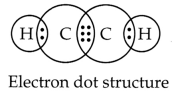
Question 31.
A solenoid is taken. It is allowed by the flow of an electric
current. Raju observes the pattern of the magnetic field due to the
current-carrying solenoid. Answer the following questions based on his
observations.
(a) State the factors on which the strength of an electromagnet
depends.
(b) Why did the television get a dark patch when the magnet was
brought near its screen?
Answer:
(a) Factors affecting the strength of an
electromagnet:
- The number of turns in the coil If the number of turns in the coil is increased, the strength of the electromagnet increases.
- The current flowing in the coil If the current in the coil is increased, the strength of the electromagnet increases.
- The length of the air gap between the poles If the length of the air gap between the poles of an electromagnet, decreases then its strength increases. (2)
(b) The television has an electromagnet installed in it. When a magnet is brought closer to the screen, the two magnetic field lines interfere and spoil its functioning. (1)
Question 32.
(i) Write two points of difference between electrical energy
and electric power.
(ii) Out of 60 W and 40 W lamps, which one has a higher
electrical resistance when used?
(iii) What is the commercial unit of
electrical energy? Convert it into joules.
Answer:
(i)
| S. No. | Electric energy | Electric power |
| 1. | Electrical energy consumed by an electrical appliance is the product of its power rating and the time it is used. | Electric power is the rate at which electrical energy is consumed. |
| 2. | It is measured in kWh. | It is measured in watt or kilowatt. |
(ii) We know, Power (P) = \(\frac{\mathrm{V}^2}{\mathrm{R}}\)
Therefore, P
is inversely proportional to R as voltage remains the same.
40 W lamp has a
higher resistance.
(iii) The commercial unit of electrical energy is kWh.
⇒ 1 kWh = 1000 W ×
1 hr = 1000 W × 3600s
⇒ = 36 × 105 J
⇒ = 3.6 × 106
J
Question 33.
A motorcycle rider without a helmet met with an accident and
suffered a spinal cord injury. In this case, which signals will get disrupted
and why?
Answer:
In case of a spinal cord injury, signals for reflex
action and involuntary action will get disturbed. Reflex action is monitored and
controlled through the spinal cord of the nervous system and not by the brain.
Nerves from all over the body meet in a bundle in the spinal cord on their way
to the brain. In case of any injury to the spinal cord, the signals coming from
the nerves as well as signals coming to the receptors will be disturbed. (3)
Section D
Questions No. 34 to 36 are Long Answer Questions.
Question 34.
(i) Describe two methods for the concentration of ores.
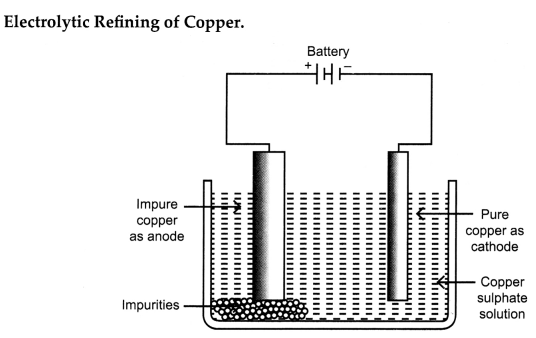
(ii) How is copper extracted from its sulphide ore? Explain the various steps
supported by chemical equations. Draw a labelled diagram for electrolytic
refining of copper.
OR
(i) Explain any two physical properties of ionic
compounds giving reasons.
(ii) List any two metals found a free state in
earth’s crust.
(pi) Metals towards the top of the activity series cannot be
obtained from their compounds by reducing with carbon. Why?
(iv) What will
you observe when:
(a) Some zinc pieces are put in the copper sulphate
solution.
(b) Some silver pieces are put into green-coloured ferrous sulphate
solution.
Answer:
Two methods used for the separation of ores are:
(i)
Froth Flotation Method: It is generally used to remove gangue from sulphide
ores. First the ore is powdered and a suspension in water is formed. To this ore
Collectors and froth stabilisers were added. The collectors generally used are
pine oils, fatty acids etc. The function of collectors is to increase the
non-wettability of the metal part of the ore and allows it to form a froth.
Froth Stabilizers (cresols, aniline etc.) sustain the froth. The oil wets the
metal and the water wets the gangue. Paddles and air constantly stir up the
suspension to create the froth. This frothy metal is skimmed off the top and
dried to recover the metal.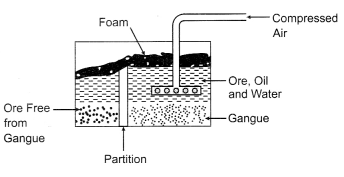
(ii) Magnetic ore Separation: This method is used in those cases where either
ore or the impurities are of magnetic nature. In this method, the powdered
impure ore in the form of a thin layer is allowed to fall on a rubber belt which
moves horizontally over two rollers, one of which has an electromagnet attached.
As the ore particles roll over the belt, the magnetic component in the ore gets
attracted towards the magnet. It gets collected in a heap while the non magnetic
component forms a separate heap.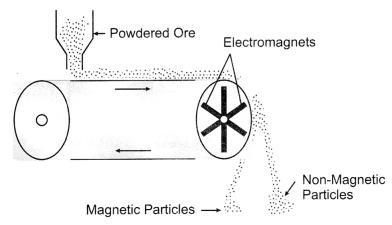
(ii) Copper is extracted from suiphide ore by roasting. It is
done in the presence of air: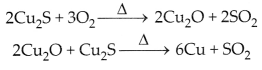
OR
(i) The two physical properties of ionic compounds
are:
1. Ionic compounds are usually crystalline solids because their
oppositely charged ions attract one another strongly and forms a regular crystal
structure.
2. Ionic compounds have high melting and boiling points because
ionic compounds are composed of oppositely charged positive and negative ions
held together by a strong electrostatic force of attraction. Therefore, a large
amount of energy is required to overcome these forces.
(ii) Gold and Platinum are the two metals that are found in a free state in the earth’s crust. These metals are located at the bottom of the activity series.
(iii) Metals such as sodium, magnesium, calcium, and aluminium high up in the reactivity series are very reactive and cannot be obtained from their compounds by heating with carbon. This is because these metals have more affinity for oxygen than carbon.
(iv) (a) The blue solution will become colourless, and reddish-brown copper
metal will be deposited.
(b) When some silver pieces are put into the green coloured
ferrous sulphate solution, there will be no reaction because Ag is less reactive
than the iron:
Ag(s) + FeSO4(aq) → No reaction
Question 35.
(a) ‘Plants also perform chemical coordination’.
Elaborate.
(b) Name various plant hormones. Also give their physiological
effects on plant growth and development.
Or
(a) Explain how traits are
controlled by genes only.
(b) Give an example in which both genes exist
independently of each other in humans.
Answer:
(a) Plants also perform
chemical coordination for various activities with the help of hormones. These
are the chemical compounds released by stimulated cells that diffuse to various
locations in plants performing different functions. These hormones produced by
plants are also called phytohormones. (2)
(b) Different types of hormones
produced by plants are Auxin, Gibberellins, cytokines, Abscisic acid, and
Ethylene. (3)
| Plant Hormone | Physiological Effect |
| Auxin | Synthesized in the young tip of roots and shoots. It diffuses towards the shady side of the plant, which stimulates the cells to grow longer, resulting in the bending of the shoot towards the light. |
| Promotes cell elongation and division. | |
| Plays an important role in the formation of roots and seedless fruits. | |
| Gibberellins | Helps in the growth of the stem and flower. |
| Helps in the germination of seeds. | |
| Cytokinins | Promote cell division and delay leaf aging. |
| Also, stimulates leaf expansion. | |
| Abscisic acid | Growth inhibitor |
| Reverses the growth-promoting effects of auxins and gibberellins. | |
| Promotes transverse growth. | |
| Ethylene | Essential for fruit ripening, promotes senescence and abscission of leaves. |
Or
(a) Plants have hormones that can trigger growth. If the hormone works
efficiently, a lot of hormones will be made (i.e., tall plant).
If the gene
for the enzyme has alteration, the enzyme will not be efficient. Hence, the
amount of hormone produced will be less (i.e., small plant). This proves that
the traits (characters) are controlled by genes only. (2)
(b) Both genes
exist independently of each other in humans can be explained by the codominance
phenomenon of blood groups. There are four types of blood groups A, B, AB, or 0,
and controlled by genes IA, IB, and IO. The
genes IA and IB show codominance because both express
themselves independently as shown.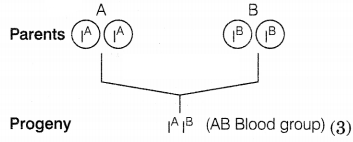
Question 36.
Describe the significance of spore formation in Rhizopus.
OR
What is vegetative propagation in plants? Describe the process of
vegetative propagation through leaves in plants?
Answer: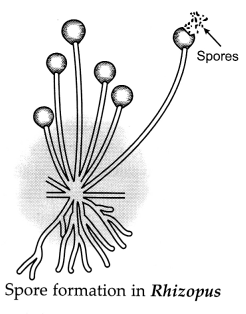
Rhizopus is a fungus that commonly grows on bread, pickle and
jam when conditions are favourable for its growth. It is a simple multicellular
organism but shows specific reproductive parts. The thread like structure that
can be seen on moist left-over bread pieces are the hyphae of the bread mould.
They are not the reproductive parts. The tiny-round headed structures on a thin
stalk are the . reproductive parts. The round blobs are the sporangia, inside
which are a large number of tiny cells or spores that help in giving rise to the
new Rhizopus individuals. These spores have thick walls that protect them till
they find a moist surface to grow. Hence, these spores are a means of asexual
reproduction in Rhizopus.
OR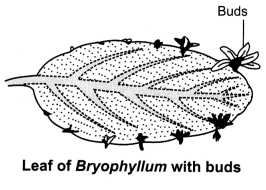
Vegetative propagation is a method of asexual reproduction in
plants where new plants are produced from the vegetative parts of plants like
root, stem and leaves. While animals cannot use this method of reproduction,
plants can. This method is used to produce new plants by layering or grafting as
in rose, jasmine, sugarcane and grapes for agriculture purposes. It has both
benefits and some drawbacks. Some examples of vegeta¬tive propagation are shown
below:
(i) Propagation by buds on leaf margins in Bryophyllum leaf. The buds
that develop along the leaf margin fall on the soil and each of them develops
into a new plant.
(ii) A small cutting of a money plant when kept in water in
a glass container or in a pot with soil grows into a new plant.
(iii) Buds in
potatoes and ginger can grow into new plants under suitable conditions.
(iv)
In sweet potatoes, the roots bear adventitious buds that can grow into new
plants under favourable conditions.
Section E
Questions No. 37 to 39 are case-based/data-based questions with 2 to 3 short sub-parts. Internal choice is provided in one of these sub-parts.
Question 37.
The table given below shows the hints given by the quiz
master in a quiz.
| Hints |
| (i) Compound ‘X’ is used in cough syrups and many tonics. |
| (ii) ‘Y’ is formed on heating ‘A’ in the presence of alk. KMnO4 |
| (iii) ‘A’ is also soluble in water in all proportions. |
Based on the above hints answer the following questions.
(a) Name the
compound X. Write its chemical formula.
(b) Which gas is evolved when the
compound X reacts with sodium? Write the chemical equation involved in the
reaction of X with sodium.
Or
Complete the following equation for X and
identify Y.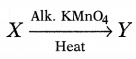
Answer:
(a) The compound X is ethanol as it is soluble in
water in all proportions and used in cough syrups. The chemical formula is
CH3CH2OH.
(b) Hydrogen gas is evolved when ethanol
reacts with sodium.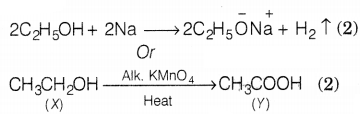
The compound ‘Y’ is ethanoic acid.
Question 38.
There are many plants in which parts like the root, stem and
leaves develop into new plants under appropriate conditions. Unlike most
animals, plants can indeed use such a mode for reproduction. This property of
vegetative propagation is used in methods such as layering or grafting to grow
many plants like sugarcane, roses, or grapes for agricultural purposes. Plants
raised by vegetative propagation can bear flowers and fruits earlier than those
produced from seeds. Such methods also make possible the propagation of plants
such as banana, orange, rose and jasmine that has lost the capacity to produce
seeds. Another advantage of vegetative propagation is that all plants produced
are genetically similar enough to the parent plant to have all its
Characteristics.
1. Take a potato and observe its surface. Can notches be seen?
2. Cut the
potato into small pieces such that some contain a notch or bud and some do
not.
3. Spread some cotton on a tray and wet it. Place the potato pieces on
this cotton. Note where the pieces with the buds are placed.
4. Observe
changes in these potato pieces over the next few days. Make sure that the cotton
is kept moistened.
(a) Which parts of plants are used for vegetative
propagation?
(b) Give any one advantage of vegetative propagation.
(c)
Give an example of a plant that shows vegetative propagation.
OR
What
observation can be seen in the above activity?
Answer:
(a) There are many
plants in which parts like the root, stem, and leaves are used for vegetable
propagation.
(b) The advantage of vegetative propagation is that all plants produced are genetically similar em to the parent plant to have all its characteristics.
(c) Rose shows vegetative propagation.
OR
The potato pieces having buds
gradually grow and develop. But there is no growth and development in potato
pieces without bud.
Question 39.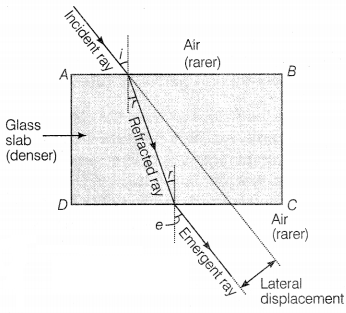
The above figure shows a glass slab at which an incident ray falls at an angle
of incidence i. The emergent ray is coming from the glass slab at an angle of
emergence e. The refractive index of the glass slab is 1.5. The speed of light
in air is 3 × 108 m/s.
(a) Based on the text and data given in the
above paragraph, what is the angle between incident ray and emergent ray?
(b)
Which quantity remains constant when a light travels from air to a glass
slab?
(c) What is the speed of light in the glass slab?
Or
What will
happen when incident light falls normally to the surface of the glass slab?
Answer:
(a) As the incident ray and the emergent ray are parallel to each
other. So, the angle between them is zero. (1)
(b) When the light travels
from one medium to another medium, then the frequency of the light remains
constant. (1)
(c) The speed of light is air, c = 3 × 108 m/s
The speed of light in the glass, v =?
Refractive index of glass,
µg = 1.5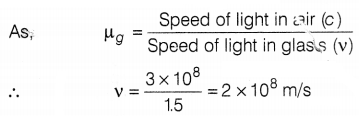
Or
When the incident ray falls normally to the surface of the glass slab,
there is no bending of the ray of light, it goes straight without any deviation.
(2)