CBSE Sample Papers for Class 10 Science Set-8
Class 10thCBSE Sample Papers for Class 10 Science Set-8
CBSE Sample Papers for Class 10 Science Set 8
Time: 3 Hours
Maximum Marks: 80
Instructions
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
- Section A consists of 20 objective-type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
- Section D consists of 3 Long Answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 4 marks each with sub-parts.
Section A
Select and write the most appropriate option out of the four options given for each of the questions 1-20.
Question 1.
The given experiment shows the reaction between the iron nail
and copper sulphate. It produces a green colour solution. The products obtained
are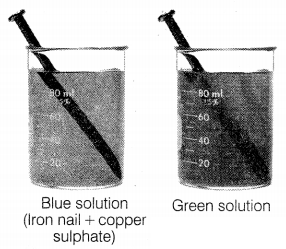
(a) Ferric sulphate, copper
(b) Cuprous sulphate, iron
(c) Iron sulphate, cupric sulphate
(d) Ferrous sulphate, copper
Answer:
(d) Ferrous sulphate, copper
Iron and copper sulphate reacts to
form ferrous sulphate and copper.![]()
This reaction is known as displacement reaction.
Question 2.
If you focus the image of a distant object, whose shape is
given below, on a screen using a convex lens. The shape of the image of this
object on the screen would be :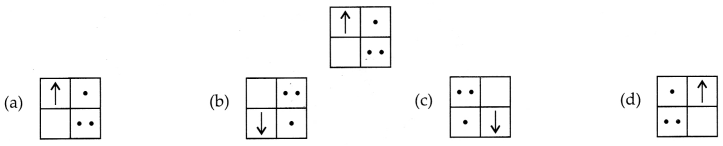
Answer: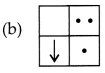
Explanation: Since, the image formed is inverted for distant
objects.
Question 3.
Galvanisation is a method of protecting iron from rusting by
coating it with a thin layer of
(a) gallium
(b) aluminium
(c) zinc
(d) Silver
Answer:
(c) zinc
Galvanization is a method of protecting
iron from rusting by coating it with a thin layer of zinc (Zn) metal.
Question 4.
During deficiency of oxygen in tissues of human beings pyruvic
acid is converted into lactic acid in:
(a) Cytoplasm
(b) Chloroplast
(c) Metochondria
(d) Golgi body
Answer:
(b) Cytoplasm
Explanation:
The pyruvate produced during glycolysis usually enters the Kerb’s cycle as
acetyl coenzyme A in the mitochondrial matrix, where it provides a reservoir of
chemical energy (ATP, NADH and FADH2). Pyruvic acid can be
transformed to lactic acid as one of its potential fates in cellular
respiration. Under stressful conditions, this often occurs in the cytoplasm of
muscle tissue.
Question 5.
Pooja studied the metals. She came to know about its many
properties. Which of the following is a characteristic of metals?
(a) They
have one to three valence electrons
(b) They have 4 to 8 valence
electrons
(c) They are brittle
(d) They are capable of forming anions
easily
Answer:
(a) They have one to three valence electrons
Metal can
easily give their electrons and form electro-positive ions. They have one to
three valence electrons in their valence shell, They are not brittle and do not
form anions.
Question 6.
Which of the following statements accurately describes the V –
I graphs plotted by a student for three samples of nichrome wire with
resistances R1, R2, and R3?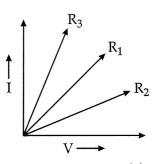
(a) R1 = R2 = R3
(b)
R1 > R2 > R3
(c) R3 >
R2 > R1
(d) R2 > R1 >
R3
Answer:
(d) R2 > R1 >
R3
Explanation: As it is clear from the graph, the current for the
A2 conductor is less than A1 and A1 is less
than A3 we can say IA2 < IA1 <
IA3.
We know
R = \(\frac{\mathrm{V}}{\mathrm{I}}\)
or R ∝
\(\frac{1}{\mathrm{I}}\)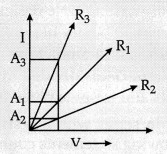
If I is less, then R will be more.
IA2 <
IA1 < IA3.
R2 > R1 >
R3.
Question 7.
Which of the following is not a straight-chain
hydrocarbon?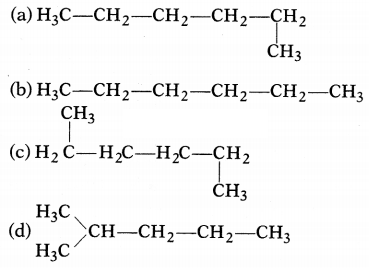
Answer: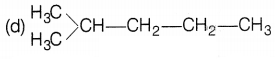
It is a branched chain hydrocarbon and not a straight chain
hydrocarbon. Rest three are straight-chain hydrocarbons.
Question 8.
Multiple fission is a reproductive strategy observed in
certain organisms. Which of the following statements accurately describes
multiple fission?
(a) Multiple fission is a type of asexual reproduction
where a single-parent organism divides into multiple offspring
simultaneously.
(b) Multiple fission is a type of sexual reproduction where
two parent organisms fuse their genetic material to produce multiple
offspring.
(c) Multiple fission is a process where a single-celled organism
divides into two identical daughter cells.
(d) Multiple fission is a process
where multiple cells fuse to form a larger, multicellular organism.
Answer:
(a) Multiple fission is a type of asexual reproduction where a
single-parent organism divides into multiple offspring simultaneously.
Explanation: Multiple fission is a reproductive strategy commonly observed in
certain unicellular l organisms such as protists and some bacteria. This process
involves the parent cell dividing into I multiple daughter cells, each of which
develops into a new individual organism. The offspring produced through multiple
fission are genetically identical to the parent organism. This reproductive
strategy allows for rapid population growth and is advantageous in environments
with favorable conditions for survival and growth.
Question 9.
Energy in the case of higher plants and animals is obtained
by
(a) breathing
(b) tissue respiration
(c) organ respiration
(d)
digestion of food
Answer:
(b) tissue respiration
Tissue respiration is
the exchange of oxygen and carbon dioxide between different tissues of plants
and animals.
Question 10.
A student connects an ammeter in series with a resistor in a
circuit and observes that the ammeter reading is zero. Which of the following
statements could explain this observation?
(a) The resistor is not
functioning properly.
(b) The ammeter is faulty or not connected
correctly.
(c) There is no electric current flowing through the circuit.
(d) The ammeter has reached its maximum reading and cannot measure higher
currents.
Answer:
(c) There is no electric current flowing through the
circuit.
Explanation: An ammeter is designed to measure the electric current
passing through a circuit. When an ammeter reading is zero, it suggests that
there is no electric current flowing through the circuit In this scenario, if
the ammeter is correctly connected in series with a resistor and the reading is
zero, it indicates that there is no current flowing through the circuit.
Possible reasons for this inch an open switch, disconnected wires, or a power
source that is not providing a voltage difference.
Question 11.
The diagram shows the central nervous system, which has been
blocked in three different places by a drug used as an anesthetic.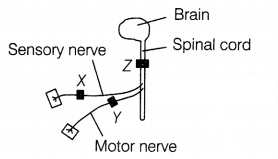
Three men had one anesthetic block at X, Y, and Z
respectively. One of the men can move his leg in response to a pinprick but does
not feel it. Where is the anesthetic block in this man?
(a) At X
(b) At
Y
(c) At Z
(d) No block
Answer:
(c) At Z
Movement of the leg in
response to a pinprick is a reflex action. Reflex action is controlled by the
spinal cord and does not involve any thinking, hence brain does not play any
role.
Question 12.
The proper representation of a series combination of cells
for obtaining maximum potential is: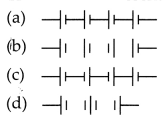
Answer:![]()
Explanation: The maximum potential is obtained when cells are
connected in series such that the negative terminal of the first cell is
connected to the positive terminal of the second cell and so on. E.g.,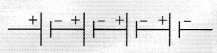
Question 13.
The following figures show the path of light rays through
three lenses marked L1, L2, and L3 and their
focal points F1, F2, and F3,
respectively.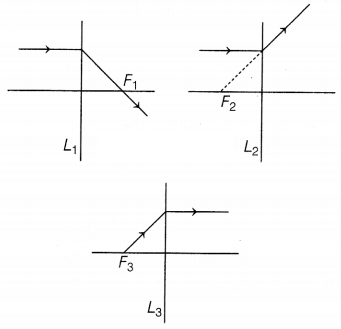
Out of L1, L2, and L3 concave lens/lenses
is/are
(a) only L4
(b) only L2
(c) only
L3
(d) Both L1 and L3
Answer:
(b) only
L2
A concave lens is a diverging lens. It diverges the parallel
beam of light rays (as shown in the figure)
The ray parallel to the principal axis is getting diverged in
the case of lens L2 only. So, only lens L2 is a concave
lens.
Question 14.
Which of the following statements is true about binary
fission?
(a) Some multicellular organisms also reproduce through binary
fission.
(b) Binary fission produces two new organisms.
(c) Binary fission
in Amoeba happens only in the vertical plane.
(d) Binary fission in
Leishmania can happen in any plane.
Answer:
(b) Binary fission produces
two new organisms.
Explanation: Only unicellular organisms reproduce through
binary fission. In binary fission, a unicellular organism (a cell) divides to
form two unicellular organisms (two cells). Binary fission in Amoeba can happen
in any plane. Binary fission in Leishmania happens in a definite orientation
(plane) to the body because Leishmania has a somewhat organised structure.
Question 15.
Which statement is true for a dominant allele?
(a) It
cannot undergo mutation
(b) It gives a greater chance of survival than a
recessive allele
(c) It gives the same phenotype in heterozygotes and
homozygotes
(d) It is only responsible for male characteristics
Answer:
(c) It gives the same phenotype in heterozygotes and homozygotes
Dominant allele always expresses itself whether present in heterozygous or
homozygous conditions. (Excluding exceptions).
Question 16.
Which of the following trait is not controlled by genes?
(a) Eye colour
(b) Height
(c) Hair colour
(d) None of these
Answer:
(d) None of these
Explanation: Characters or traits are the
characteristics that a person expresses and can be seen in their phenotype.
Height, eye colour, and hair colour are all controlled by genes and can be
inherited.
Direction (Q. Nos. 17-20) consists of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both
A and R are true but R is not the correct explanation of A.
(c) A is true but
R is false.
(d) A is false but R is true.
Question 17.
Assertion (A): Acetic acid is more acidic than alcohol.
Reason (R): The ion formed after the removal of a proton from acetic acid is
less stable.
Answer:
(c) Assertion is true, but Reason is false.
Acetic
acid is more acidic than alcohol because of the stability of ions formed after
the removal of a proton.
Question 18.
Assertion: Voltmeter is always connected in parallel across
the circuit while measuring the potential difference.
Reason: As the voltage
in parallel circuits is measured to be the same.
Answer:
(a) Both A and R
are true and R is the correct explanation of A
Explanation: The voltmeter is
indeed always connected in parallel across the circuit while measuring the
potential difference. This is because, in a parallel circuit, the voltage across
each component is ? the same.
The Reason also provided correctly explains why the voltmeter is connected in parallel. By connecting the voltmeter in parallel, we ensure that it measures the same potential difference as the component we are interested in.
Question 19.
Assertion (A): Fertilisation is a unique feature in
flowers.
Reason (R): It is followed by pollination.
Answer:
(c) A is
true, but R is false.
Fertilization is a unique feature of flowers because
the male gamete released by pollen is involved in fertilization. The male gamete
unites with the egg and this forms a zygote. It is followed by embryo formation
and not pollination.
Question 20.
Assertion: Photosynthesis is considered as an exothermic
reaction.
Reason: Photosynthesis is an endothermic reaction because sunlight
energy is absorbed by green plants during this process.
Answer:
(d) A is
False but R is true
Explanation: Photosynthesis is an endothermic reaction
because sunlight energy is absorbed by green plants during this process.
Section B
Questions No. 21 to 26 are Very Short Answer Questions.
Question 21.
Discuss the nature of covalent bonds.
Answer:
Carbon
has 4 electrons in its valence shell. To complete its octet, it either needs to
gain 4 electrons or lose 4 electrons to the other atom. Both these processes are
impossible. Therefore, the carbon atom achieves noble gas configuration by
sharing 4 electrons with other atoms of itself or atoms of other elements. The
bonds that are formed by sharing electrons are known as covalent bonds. In
covalent bonding, both atoms share the valence electrons, i.e. the shared
electrons belong to the valence shells of both atoms. CH3Cl is called
chloromethane, which contains 1 carbon atom, 3 hydrogen atoms, and 1 chlorine
atom. (1)
Question 22.
Do genetic combinations of the mother play a significant role
in determining the sex of a new born?
Answer:
No, genetic combinations of
the mother do not play a significant role in determining the sex of a new born;
because mother has only one type of sex chromosomes i.e., X chromosomes, but a
the father has two types of chromosomes X and Y chromosomes. So, all children
will inherit the X chromosome from the mother and whether X or Y, bearing sperm
from father fertilises the egg will determine the sex of new born.
Question 23.
Difference between exocrine and endocrine glands.
Or
What is a reflex arc? How do muscle cells move?
Answer:
Differences
between the exocrine gland and the endocrine gland are as follows.
| Exocrine gland | Endocrine gland |
| It does not pour its secretion into lymph or blood. | It pours its secretion into lymph or venous blood. |
| A duct is often present. | The glands are without ducts or are ductless. |
| The secretion is poured directly over the target tissue. | The secretion is transported to the target tissue through blood. |
| The secretion is enzymatic, lubricant, or excretory. | The secretion contains hormones. |
| Examples: Sweat glands, lacrimal glands, salivary glands, mammary glands, etc. | Examples: Pituitary gland thyroid gland and adrenal gland. |
Or
The pathway taken by nerve impulses in a reflex action is called the
reflex arc. They allow rapid response to a stimulus, 0 g. pulling of hand on
touching a hot object. (1)
Muscle cells have special proteins that change
their shape and arrangement in the cell In response to electrical impulses. This
forces the muscle cells to contract and relax, causing their movement. (1)
Question 24.
Give two ways in which:
(i) Biodegradable substances
affect our environment.
(ii) Non-biodegradable substances affect our
environment.
Answer:
(i) The two ways in which biodegradable substances
would affect the environment are:
- Decomposition of biodegradable substances results in the production of a foul smell.
- The area where biodegradable wastes are accumulated serves as a good breeding place for mosquitoes, flies etc. which are the main carriers of germs for diseases like cholera, jaundice, typhoid etc.
(ii) The two ways in which non-biodegradable substances would affect the environment are:
- Non-biodegradable substances like pesticides (DDT) enter the food chain and leads to biomagnification.
- As non-biodegradable substances cannot be degraded naturally so they accumulate in the soil causing pollution and also reducing the fertility of the soil.
Question 25.
Student experiments and plots the V-I graph of three samples
of nichrome wire with resistances R1, R2, and
R3, respectively as shown in the figure. Interpret the graph by
considering R1, R2, and R3 in proper
order.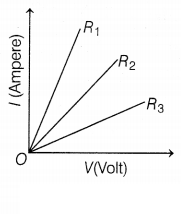
Or
The diagram shows the lengthwise section of a
current-carrying solenoid.
Indicates current entering into the page,
Indicates current emerging out of the page.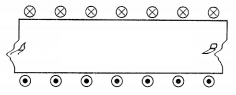
Decide which end of the solenoid AorB, will behave as the
North pole. Give a reason for your answer. Also, draw field lines inside the
solenoid.
Answer:
As we know, slope of V-I graph tells about the
resistance and (slope of V and I) ∝ \(\frac{1}{\text { resistance }}\),
i.e,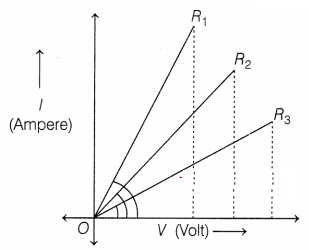
So, R3 > R2 > R1 (2)
From the diagram,
we can see that the current is entering from A and emerging from B.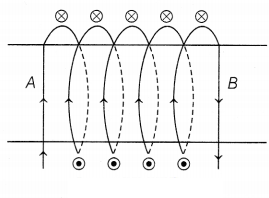
Thus, using the right-hand thumb rule, the direction of
magnetic field lines is from B to A. We know that magnetic field lines move from
North to South direction. Thus, B represents the North Pole and A represents the
South Pole. (1)
Question 26.
Write one difference between the sexual and asexual mode of
reproduction. Which species is likely to have better chances of survival, the
one reproducing asexually or the one reproducing sexually? Justify your
answer?
OR
(i) Budding, fragmentation, and regeneration, all are
considered as asexual mode of reproduction. Why?
(ii) With the help of neat
diagrams, explain the process of regeneration in Planaria.
Answer:
In
sexual reproduction, male and female gametes from two different individuals
unite, leading to variation in offsprings due to the mixing up of genetic
material whereas in case of asexual reproduction only single parent is involved
and there is no formation of gametes. The ones that are produced sexually have
been chances of survival due to mixing up of genetic material between the two
different individuals, as the an increase in genetic variations in the
offsprings which provides maximum chances for the survive species in the
changing environment as well as under unfavourable conditions. This variation in
space is also essential for evolution.
OR
(i) Budding, fragmentation and
regeneration are considered as asexual modes of reproduction because only one
parent is involved and no sex cells are involved.
(ii) Regeneration in
Planaria.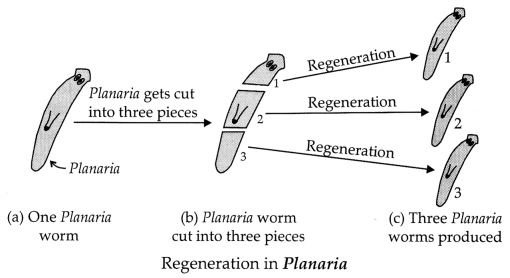
Section C
Questions No. 27 to 33 are Short Answer Questions.
Question 27.
Cheshta, a 10th-class student was asked to identify iron,
copper, zinc, and aluminium only by observing the effect of the action of
concentrated nitric acid and caustic soda on each metal. How did she put the
reactions of these metals with each of the reagents?
Or
Compound ‘X’ and
aluminium are used to join railway tracks. Identify the compound ‘X’ and name
the reaction. Write the equation for it.
Answer:
| The action of Concentrated Nitric Acid | Action of Caustic Alkali | Inference |
| No characteristic change | No characteristic change | Iron |
| Liberates brown-coloured NO2 gas | No characteristic change | Copper |
| Liberates brown-coloured NO2 gas | Liberates hydrogen gas | Zinc |
| No characteristic change | Liberates hydrogen gas | Aluminium |
Or
X is Fe2O3, i.e. iron (III) oxide.
The
reaction involved is thermite reaction or aluminothermy.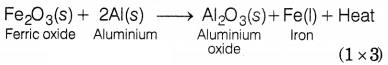
Question 28.
(i) Define catenation. Why no other element exhibits the
property of catenation to the extent seen in carbon compounds?
(ii) What do
you mean by homologous series? Give the general formula of the homologous series
of alkenes?
(iii) What do you mean by isomers? Give an example.
Answer:
(i) Carbon can link with carbon atoms via covalent bonds to form long
chains, branched chains and closed ring, called catenation. Carbon shows
catenation to a large extent because of its small size and having C – C strong
bonding.
(ii) A homologous series is a group of organic compounds having similar
structures and similar chemical properties in which the successive compounds
differ by CH2 group.
The general formula of the homologous series
of alkenes is CnH2n where n is the number of carbon atoms
in one molecule of alkene.
(iii) The organic compounds having the same molecular formula but different structures are known as isomers. n-butane and iso-butane are two isomers of butane.
Question 29.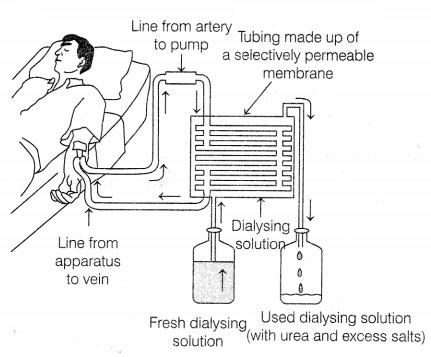
(a) Identify the process taking place in the above diagram. When does this
process become essential for an individual?
(b) Describe the above process in
detail.
Answer:
(a) The process is called dialysis. This process is
essential for an individual who is suffering from complete renal failures, i.e.
both kidneys are damaged due to an infection, injury, high BP, etc. (1)
(b) The dialysis machine, also known as an artificial kidney, contains several tubes with a semi-permeable lining suspended in a tank filled with a dialyzing fluid. This fluid has the same osmotic pressure as that of blood except, it is devoid of nitrogenous wastes such as urea. During the procedure of dialysis, the patient’s blood is passed through these tubes.
As the blood passes, the waste products from the blood move into dialyzing fluid by diffusion, and the purified blood is pumped back into the patient’s body. The dialysis unit allows the blood to run along one side of a cellophane membrane and dialysis fluid in opposite directions. This is generally done to maintain the concentration gradient between the patient’s blood and the dialysis fluid. (2)
Question 30.
Define the following terms concerning spherical mirrors:
(i) Pole
(ii) Centre of curvature
(iii) Principal focus
(iv) Principal
axis
(v) Radius of curvature
(vi) Aperture
Answer:
(i) Pole: It is
the centre of the reflecting surface of the mirror.
(ii) Centre of curvature: It is the centre of the hollow sphere of which the reflecting surface of the mirror is a part.
(iii) Principal focus: Incident rays parallel to the principal axis, after reflection, either converge to or appear to diverge from a fixed point on the principal axis. This point is called the principal focus of a spherical mirror.
(iv) Principal axis: It is the straight line passing through the pole and centre of curvature of a spherical mirror.
(v) Radius of curvature: The radius of curvature of a spherical mirror is the radius of the hollow sphere of glass of which the mirror is a part.
(vi) Aperture: The portion of a mirror from which the reflection of light actually takes place is called the aperture of the mirror.
Question 31.
The diagram shown below is the connection of
(a) 1 Ω and 2
Ω resistors in series.
(b) 12 Ω and 2 Ω resistors in parallel.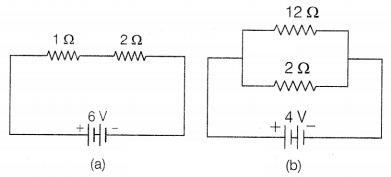
Compare the power used in 2 Ω resistor in each of the following circuits.
(a)
A 6V battery in series with 1 Ω and 2 Ω resistors,
(b) A 4V battery in
parallel with 12 Ω and 2 Ω resistors.
Answer:
(a) The circuit is shown in
lift;
Resistances are connected in series combination.
Current in the
circuit, I = \(\frac{V}{R_1+R_2}=\frac{6}{3}\) = 2 A
∴ Power used =
I2R
= (2)2 × 2
= 2 × 2 × 2
= 8W (1)
(b) The
circuit is shown on right,
In parallel combination, the potential across each
resistor is the same and equal to the potential applied to the circuit.
The
potential across 2 Ω resistor, V = 4V
Power used = \(\frac{V^2}{R}=\frac{4
\times 4}{2}\) = 8W
The power used in both the cases is same. (2)
Question 32.
State the reason for the following:
(i) Lemon is used for
restoring the shine of tarnished copper vessels.
(ii) Copper is used to make
hot water tanks and not steel (an alloy of iron).
(iii) Copper wires are used
in electrical appliances.
Answer:
(i) When copper vessels are exposed to
moist air, they form a green coating of basic copper carbonate
[CuCO3. CU(OH)2]. The sour substance such as lemon juice
contain acid. Lemon juice contains citric acid. This acid dissolve the coating
of copper oxides or basic copper carbonate present on the surface of tarnished
copper vessels and make them shining red-brown again.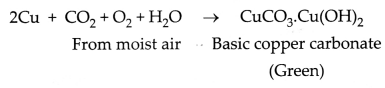
(ii) Copper does not react with cold water, hot water, or
steam. However, iron reacts with steam. If the hot water tanks are made of steel
(an alloy of iron), then iron would react vigorously with the steam formed from
hot water to corrode the tank due to the formation of iron oxide.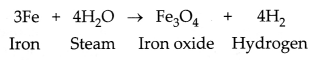
Therefore, copper is used to make hot water tanks and not
steel.
(iii) Copper metal has high melting and boiling point, good conductor of electricity and can be drawn into thin wires. This is the reason behind the use of copper metal to make electrical wires.
Question 33.
State whether an a-particle will experience any force in a
magnetic field, if (α-particles are positively charged particles)
(a) it is
placed in the field at rest.
(b) it moves in the magnetic field parallel to
field lines.
(c) it moves in the magnetic field perpendicular to field
lines.
Justify your answer in each case.
Answer:
(a) No, it will not
experience any force. A magnetic field exerts force on a moving charged particle
only. (1)
(b) No, it will not experience any force because the magnetic field
exerts a force in a perpendicular direction to the motion of the particle.
(1)
(c) Yes, it will experience a force in a direction perpendicular to the
direction of its motion and the direction of the magnetic field can be
determined by Fleming’s left-hand rule. (1)
Section D
Questions No. 34 to 36 are long answer questions.
Question 34.
During the reaction of some metals with dilute hydrochloric
acid, the following observations were made by a student:
(i) Silver does not
show any change.
(ii) Some bubbles of a gas are seen when lead is reacted
with the acid.
(iii) The reaction of sodium is found to be highly
explosive.
(iv) The temperature of the reaction mixture rises when aluminium
is added to the acid.
Explain these observations giving appropriate
reasons.
OR
Write the name, method of preparation and uses of the
following:
(i) CaOCl2
(ii)
CaSO4.\(\frac{1}{2}\)H2O
(iii) NaHCO3
Answer:
(i) Silver is covered with a thin layer of silver chloride, so it
does not react with dilute hydrochloric acid.
(ii) Bubbles of hydrogen gas are evolved when the lead is
reacted with the acid.
(iii) The reaction of sodium is found to be highly explosive
because sodium is a highly reactive metal. It reacts with hydrochloric acid
explosively forming hydrogen gas along with the release of large amount of
heat.
(iv) The temperature of the reaction mixture rises when
aluminium is added to the acid because the reaction is highly exothermic in
nature.
OR
CaOCl2: Its chemical name is calcium
oxychloride. It is also known as bleaching powder. Preparation: Bleaching powder
is produced by the action of chlorine on dry slaked lime.
Ca(OH)2
+ Cl2 → CaOCl2 + H2O
Uses: It is used as a
bleaching agent in the textile industry.
(ii) CaSO4.\(\frac{1}{2}\)H2O: It is calcium sulphate hemihydrate. It is also known as Plaster of Paris.
Plaster of Paris is prepared by heating\(\frac{1}{2}\)Gypsum at 373K.
CaSO4.2H2O → CaSO4.\(\frac{1}{2}\)H2
+ \(\frac{3}{2}\)H2
Uses: It is used to join bones, buildings and
in dentistry.
(iii) NaHCO3: Its chemical name is sodium hydrogen carbonate
(NaHCO3). Also known as baking sodium
Preparation:
NaCl +
H2O + CO2 + NH3 → NH4Cl +
NaHCO3
Uses: It is used in the food industry and bakery, as an
antacid and mild antiseptic.
Question 35.
Differentiate between the following.
(a) Pollen tube and
style
(b) Fission in Amoeba and Plasmodium
(c) Fragmentation and
regeneration
(d) Bud of Hydra and Bryophyllum
(e) Vegetative propagation
and spore formation
Or
(a) Hormones are needed by our body in an
appropriate amount, slightly more or less secretion causes disorders in our
body. Illustrate this by using three examples.
(b) Why do we call the
pituitary gland a master gland? Where is it located?
Answer:
(a)
Differences between pollen tube and style are
| Pollen Tube | Style |
| A tube growing out of pollen grain when it reaches the stigma. | The middle elongated part of the carpel, i.e. female part of a flower. |
| It transports male gametes from pollen grains to ovules. | The attachment of stigma to the ovary. |
(b) Fission in Amoeba is binary and in Plasmodium is multiple. The difference is
| Binary Fission | Multiple Fission |
| The parental body divides into two identical daughter cells at a time. | The parental body divides into numerous daughter cells simultaneously. |
(c) Differences between fragmentation and regeneration is
| Fragmentation | Regeneration |
| The method in which a multicellular organism breaks up into two or more smaller fragments. | The growth of a whole new organism from any of its body parts, i.e. single segment forming a new individual. |
(d) Differences between the bud of Hydra and Bryophyllum is
| Bud of Hydra | Bud of Bryophyllum |
| It is seen during budding as an outgrowth on the body of Hydra, which gets fully grown and then detaches from the body and becomes a new individual. | It is present on the leaf margins of the leaf of Bryophyllum and develops into a new plant when it comes in contact with soil and other favourable conditions. |
(e) Differences between vegetative propagation and spore formation is
| Vegetative Propagation | Spore Formation |
| New plants are obtained from different parts of the parent body like leaves, stems, etc. | Spores when fall on land, can germinate and produce new fungal colonies under favourable conditions. |
Or
(a) Hypersecretion (more secretion) or hyposecretion (less secretion)
of different hormones leads to various disorders In our body. The three common
examples are
(i) Goitre: Iodine acts as the necessary component for the
synthesis of thyroxine hormone from the thyroid gland. This disorder is caused
due to the deficiency of iodine that leads to the hyposecretion of
thyroxine.
(ii) Gigantism and dwarfism: Hypersecretion of growth hormone
results in gigantism (very tall individual). On the contrary, the hyposecretion
or deficiency of growth hormone at an early stage of life makes the person very
short, i.e. causes dwarfism.
(iii) Diabetes mellitus: Insulin secreted by the
pancreas helps to lower the blood glucose level. When it is secreted in less
amount, the body suffers from diabetes.) (3)
(b) The pituitary gland secretes
several hormones that regulate various functions of the body. It also controls
the functioning of the other endocrine glands. Hence, it is called as master
gland. The pituitary gland is located just below the hypothalamus at the base of
the brain. (2)
Question 36.
Due to gradual weakening of ciliary muscles and diminishing
flexibility of the eye lens, a certain defect of vision arises. Write the name
of this defect. Name the type of lens required by such persons to improve their
vision. Explain the structure and function of such a lens.
OR
Answer the
following questions:
(a) Draw a figure which show the arrangement for
observing the phenomenon of scattering of light in the laboratory.
(b) What
colour would you observe in the experiment? Why?
Answer:
The defect caused
due to gradual weakening of ciliary muscles and diminishing flexibility of the
eye lens is presbyopia. Presbyopia is the defect of the eye in which a person
cannot see nearby objects comfortably and distinctly without corrective eye
glasses. A presbyopic eye has a near point greater than 25 cm and is gradually
increases as the eye becomes older. The type of lens required by such a person
to improve the vision is bifocal lens.
A bifocal lens consists of both convex lens and concave lenses. The convex
lens used in the bifocal lens to correct Hypermetropia (far-sightedness) and the
concave lens is used to correct myopia (short sightedness).
OR
(a) An
arrangement for observing the scattering of light in the laboratory is as shown
below.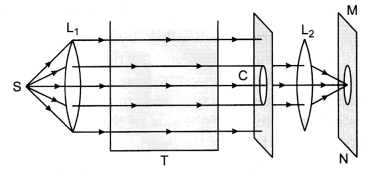
(b) 1. On the screen, first orange red colour and then bright
crimson red colour patch is observed.
2. From the other three sides of
colloidal solution of sulphur in glass tank(T), blue colour is observed. This is
because the very fine colloidal sulphur particles scatter away the blue colour
from the path of beam and only red colour (least scattered) of the beam of white
light reaches the screen through the solution.
Section E
Questions No. 37 to 39 are case-based/data-based questions with 2 to 3 short sub-parts. Internal choice is provided in one of these sub-parts.
Question 37.
The table given below shows the hints given by the quiz
master in a quiz.
| Hints |
| (i) ‘A’ is an alkali metal. |
| (ii) ‘A’ gives a compound ‘S’ (molecular mass = 40) on reacting with water. |
| (iii) On treatment with aluminium oxide ‘6’ gives a soluble compound ‘C’. |
Based on the above hints answer the following question.
(a) Identify A and
‘C’.
(b) Write all the chemical reactions (A → B, A → C)
Or
In what
forms are metals found in nature? Explain how metals react with oxygen.
Answer:
(a) A = Sodium (Na)
C = Sodium Aluminate (NaAlO2)
(2)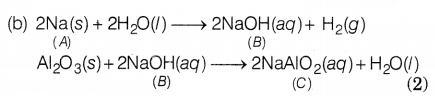
Or
Metals are found in both free and combined states.
Reaction of metals
with oxygen: All metals combine with oxygen at different rates to form metal
oxides. The general equation for this reaction is (2)
metals + oxygen → metal
oxide.
Question 38.
Some metals are found in the earth’s crust in the Free State.
Some are found in the form of the lr compounds. The metals at the bottom of the
activity series are the least reactive. They are often found* in a free state.
For example, gold, silver, platinum and copper are found in the Free State.
Copper and silver are also found in the combined state as their sulphide or
oxide ores. The metals at the top of the activity series (K, Na, Ca, Mg and Al)
are so reactive that they are never found in nature as free elements. The metals
in the middle of the activity series (Zn, Fe, Pb, etc.) are moderately reactive.
They are found in the earth’s crust mainly as oxides, sulphides or carbonates.
You will find that the ores of many metal s are oxides. This is because oxygen
is a very reactive element and is very abundant on the earth.
Thus based on reactivity, we can group the metals into the following three
categories- (i) Metals of low reactivity; (ii) Metals of medium reactivity;
(iii) Metals of high reactivity. Different techniques are to be used for
obtaining the metals falling in to each category.
(a) Why the metal sulphides
and carbonates must be converted into metal oxides prior to reduction?
(b)
Write chemical equations for the reduction of mercury from its ore Cinnabar?
(c) Why highly reactive metals such as sodium, calcium, aluminium, etc., are
used as reducing agents? Explain with an example.
OR
Write the equations
for Thermite reaction.
Answer:
(a) It is easier to obtain metal from its
oxide, as compared to its sulphides and carbonates. Therefore, metal sulphides
and carbonates must be converted into metal oxides prior to their reduction.
(b) Cinnabar (HgS) is an ore of mercury. When it is heated in air, it is
first converted into mercuric oxide (HgO). Mercuric oxide is then reduced to
mercury on further heating.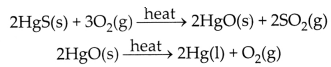
(c) Highly reactive metals, such as sodium, calcium,
aluminium, etc., are used as reducing agents because they can displace metals of
lower reactivity from their compounds. For example, when manganese dioxide is
heated with aluminium powder, the following reaction takes place.
3MnO2(s) + 4Al(s) → 3Mn(l) + 2Al2O3(S) +
Heat
OR
The reaction of iron (III) oxide (Fe2O3)
with aluminium is used to join railway tracks or cracked machine parts. This
reaction is known as the Thermite reaction.
Fe2O3(s) +
2Al(s) —> 2Fe(l) + Al2O3(s) + Heat
Question 39.
How does a metal conductor conduct electricity? You would
think that a low-energy electron has great difficulty passing through a solid
conductor. Inside a solid, the atoms are packed together with very little
spacing between them. But it turns out electrons can ‘travel’ through a perfect
solid crystal smoothly and easily, almost as if they were in a vacuum.
The motion of electrons in a conductor, however, is very different from that of charges in space. When a steady current flows through a conductor, the electrons in it move with a certain average ‘drift speed’.
One can calculate this drift speed of electrons for a typical copper wire carrying a small current and it is found to be very small of the order of 1 mms-1.
How is it then that an electric bulb lights up as soon as we turn the switch
ON? It cannot be that a current starts only when an electron from one terminal
of the electric supply physically reaches the other terminal through the bulb
because the physical drift of electrons in the conducting wires is a very slow
process.
(a) The electrons move with a certain speed inside the conductor
when a battery is connected across it, is called ___________
(b) The order of
drift speed of electrons in copper wire is ___________
(c) Do the drift speed
and thermal speed of electrons inside a metal conductor remain the same?
Or
How does an electric bulb light up as soon as we turn the switch ON?
Answer:
(a) When the battery is connected across the conductor, then a steady
current flows through the conductor and the electrons in it move with a certain
average speed called drift speed. (1)
(b) The drift speed of electrons for a
typical copper wire is found to be in the order of 1 mms-1 =
10-3 m/s. (1)
(c) No, the drift speed of electrons inside the
conductor is much smaller than its thermal velocity. (2)
Or
When we turn
the switch ON, then free electrons near the electric bulb start drifting due to
the electric field inside the conductor, hence electric current starts flowing
through the bulb as soon as we turn the switch ON. (2)