CBSE Sample Papers for Class 10 Science Set-6
Class 10thCBSE Sample Papers for Class 10 Science Set-6
CBSE Sample Papers for Class 10 Science Set 6
Time: 3 Hours
Maximum Marks: 80
Instructions
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
- Section A consists of 20 objective-type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
- Section D consists of 3 Long Answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based assessment units of 4 marks each with sub-parts.
Section A
Select and write the most appropriate option out of the four options given for each of the questions 1-20.
Question 1.
Identify gas A in the following experiment.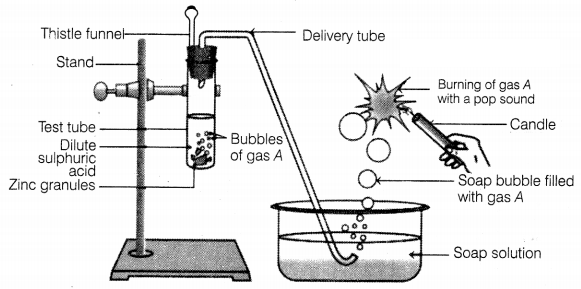
(a) Nitrogen
(b) Hydrogen
(c) Oxygen
(d) Carbon
dioxide
Answer:
(b) Hydrogen
In the given experiment, the gas evolved
(A) is hydrogen as zinc reacts with dil.H2SO4 to form zinc
chloride and hydrogen gas.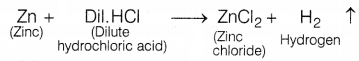
The presence of hydrogen is tested by bringing a burning
candle near the soap bubbles filled with gas A (hydrogen). In doing so, hydrogen
gas burns with a pop sound due to the reaction between hydrogen and oxygen
present in the air.
Question 2.
The colour of the solution observed after 30 minutes of
placing zinc metal to copper sulphate solution is [1]
(a) Blue
(b)
Colourless
(c) Dirty green
(d) Reddish Brown
Solution:
(b)
Colourless
Explanation: The colour of the solution observed after 30 minutes
of placing zinc metal to copper sulphate solution is colourless because zinc
being more reactive than copper displaces it from copper sulphate solution and
forms a colourless solution of zinc sulphate.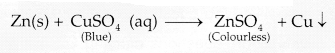
Question 3.
A sample of soil is mixed with water and allowed to settle.
The clear supernatant solution turns the pH paper yellowish-orange. Which of the
following would change the colour of this pH paper to greenish-blue?
(a)
Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer:
(d) An antacid
As pH paper turns greenish blue for weakly basic compounds and
antacids contain weak bases like Mg(OH)2. So, an antacid would change
the colour of this pH paper to greenish-blue. Other options (a) and (b) contain
acids and option (c) is a neutral salt.
Question 4.
On adding dilute sulphuric acid to a test tube containing a
metal ‘X’, a colourless gas is produced when a burning match stick is brought
near it. Which of the following correctly represents metal ‘X’? [1]
(a)
Sodium
(b) Zinc
(c) Copper
(d) Silver
Solution:
(a) Sodium
Explanation: When dilute sulphuric acid is taken in a test tube containing
sodium metal, sodium sulphate is formed along with the liberation of hydrogen
gas which is colourless gas. Thus, metal ‘X’ is sodium metal. The balanced
chemical reaction can be represented as: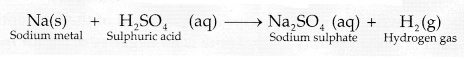
Question 5.
__________ will displace hydrogen from dilute acids.
(a)
Copper
(b) Gold
(c) Zinc
(d) Silver
Answer:
(c) Zinc
Zinc will
displace hydrogen from dilute acids as it has a higher reactivity than
hydrogen.
Question 6.
An element with atomic number ……………… will form a basic oxide.
[1]
(a) 7 (2, 5)
(b) 17 (2, 8, 7)
(c) 14 (2, 8, 4)
(d) 11 (2., 8,
1)
Solution:
(d) 11(2, 8, 1)
Explanation: Generally, metals form basic
oxide while non-metals form acidic oxide. Metals have 1 to 3 electrons in the
outermost shell whereas non-metals have 4 to 8 electrons in the outermost shell.
The electronic configuration of given elements are as follows:
| Atomic Number | Electronic Configuration |
| 7 (Nitrogen) | 2, 5 |
| 17 (Chlorine) | 2, 8, 7 |
| 14 (Silicon) | 2, 8, 4 |
| 11 (Sodium) | 2, 8, 1 |
Thus, element with atomic number 11 (sodium) having electronic configuration 2, 8, 1 is metal and it will form a basic oxide. Whereas, the other three elements having atomic numbers 7 (Nitrogen), 17 ; (Chlorine) and 14 (Silicon) are non-metals and hence they will form acidic oxide.
Question 7.
Which of the given options correctly represents the parent
acid and base of calcium carbonate?
| Parent Acid | Parent Base | |
| (a) | HCl | NaOH |
| (b) | H2CO3 | Ca(OH)2 |
| (c) | H3PO3 | CaSO4 |
| (d) | H2SO4 | CaSO4 |
Answer:
(b) Parent Acid – H2CO3, Parent Base –
Ca(OH)2
The terms ‘parent acid’ and ‘parent base’ are used to
describe the original compounds that combine to make the salt. So, calcium
carbonate salt can be obtained by the reaction between
H2CO3 (parent acid) and Ca(OH)2 (parent base)
to form calcium carbonate salt and water.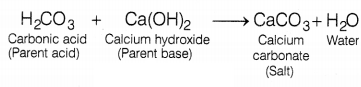
Question 8.
Generally, food is broken and absorbed within the body of
organisms. In which of the following organisms is it done outside the body?
[1]
(a) Amoeba
(b) Mushroom
(c) Paramoecium
(d) Lice
Solution:
(b) Mushroom
Explanation: The process of animals feeding on dead
and decaying substances or organisms for : getting energy, food and nutrition is
called as saprophytic nutrition and the organisms that follow saprophytic
nutrition are called saprotrophs. They are the recycler of nutrients as they
release specific enzymes that act on complex organic matter and break them into
smaller and simpler particles that are easily consumable by the organism.
Organisms like – Mushrooms, Yeast, and Bread moulds are saprophytic. They break
down food outside the body and absorb the simpler digested particles. Hence,
from the given options fungi, mushroom break down food outside the body and
absorb the simpler digested particles.
Question 9.
Iodine is necessary for the synthesis of which hormone?
(a)
Adrenaline
(b) Thyroxine
(c) Auxin
(d) Insulin
Answer:
(b)
Thyroxine
Iodine is necessary for the synthesis of thyroxine hormone.
Question 10.
A farmer wants to grow banana plants genetically similar
enough to the plants already available in his field. Which one of the following
methods would you suggest for this purpose? [1]
(a) Regeneration
(c)
Vegetative propagation
(b) Budding
(d) Sexual reproduction
Solution:
(c) Vegetative propagation
Explanation: Plants that are
genetically similar enough can be grown by means of vegetative propagation. This
method of asexual reproduction is a method of rapid propagation where the sex
cells are not involved. The new plant formed is also genetically similar to the
parent plant. In this type of asexual reproduction, parts of the plant like
stem, roots or leaves can be used to grow new plants. Banana propagation is a
type of vegetative reproduction.
Question 11.
The figure given below shows three stages in the cardiac
cycle.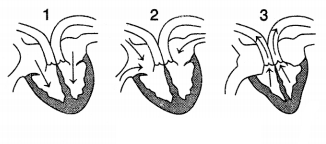
Which of the following sequences is correct regarding
this
(a) 1, 2, 3
(b) 2, 1, 3
(c) 2, 3, 1
(d) 3, 1, 2
Answer:
(b) 2, 1, 3
In Figure (2), blood is entering into the right auricle through
the superior and inferior vena cava and blood is entering into the left auricle
through the pulmonary vein. Figure (1) shows the movement of blood from the
auricles into the ventricles. Figure (3) shows the movement of blood from the
right ventricle into the pulmonary artery and from the left ventricle into the
aorta.
Question 12.
A sportsman, after a long break of his routine exercise,
suffered muscular cramps during a heavy exercise session. This happened due to:
[1]
(a) lack of carbon dioxide and formation of pyruvate.
(b) presence of
oxygen and formation of ethanol.
(c) lack of oxygen and formation of lactic
acid.
(d) lack of oxygen and formation of carbon dioxide.
Solution:
(c)
lack of oxygen and formation of lactic acid.
Explanation: During heavy
exercise, the energy demand is high but the supply of oxygen to produce energy
is limited. Therefore, anaerobic respiration takes places in the muscles cells
to fulfil the energy demand. This anaerobic breakdown of glucose leads to the
formation of lactic acid in muscles. The accumulation of lactic acid in muscles
leads to muscle cramps. Hence, due to lack of oxygen and formation of lactic
acid, a sportsman suffered muscular cramps during a heavy exercise session.
Question 13.
What is the maximum resistance that can be made using five
resistors each of \(\frac{1}{5}\) Ω?
(a) 1 Ω
(b) 5 Ω
(c) 2 Ω
(d) 2.5
Ω
Answer:
(a) 1 Ω
The maximum resistance is obtained when resistors are
connected in a series combination. Thus, equivalent resistance,
Rs
= n × R = 5 × \(\frac{1}{5}\) = 1 Ω
where, Rs = equivalent
resistance for series combination. (1)
Question 14.
When light enters the atmosphere it strikes extremely fine
particles, which deflect the rays of light in all possible directions, This is
due to – [1]
(a) reflection of light
(c) scattering of light
(b)
atmospheric refraction
(d) dispersion of light
Solution:
(c) scattering
of light
Explanation: The earth’s atmosphere is a heterogeneous mixture of
minute particles like smoke, tiny water droplets, suspended particles of dust,
and molecules of air. When a beam of light enters the atmosphere it strike on
such extremely fine particles, which deflect the rays of light in all possible
direction due to scattering of light. As a result of this phenomenon, the path
of the beam becomes visible.
Question 15.
During a Mendelian experiment, cross-breeding is done between
tall pea plants bearing violet flowers and dwarf pea plants with white flowers.
The F1 generation produced all violet flowers but half of them were
short. What can be the genotype of the tall parent?
(a) TTww
(b) TTWW
(c) TtWW
(d) TtWw
Answer: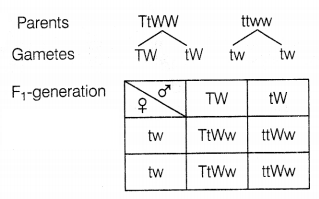
Since all the flowers are purple so tall parent is homozygous
dominant for this character. Half of the plants are tall and half are dwarf, so
a tall parent is heterozygous dominant for this character. Thus, the genotype of
tall parent-TtWW.
Question 16.
Which of the following features relates to biodegradable
substances? [1]
(a) Broken down by biological processes
(b) Remain
inert
(c) Persist in the environment for a long time
(d) May harm the
ecosystem
Solution:
(a) Broken down by biological processes
Explanation: The substances that can be easily broken down by biological
processes are called biodegradable wastes. These substances are decomposed
through the actions of fungi, bacteria, and other living organisms. For example
Food waste, paper, wood, cloth, cow-dung, human and animal excreta, etc.
On the other hand, the substances that cannot be broken down by biological processes are called non-biodegradable wastes. These substances may be in solid, liquid or gaseous form. These substances are inert and simply persist in the environment for a long time or may harm the various members of the ecosystem. For example, DDT, insecticides, pesticides, mercury, plastics, polythene bags, glass, and radioactive wastes. These non-biodegradable wastes are major pollutants of the environment.
Directions (Q.Nos. 17-20) consist of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both
A and R are true but R is not the correct explanation of A.
(c) A is true but
R is false.
(d) A is false but R is true.
Question 17.
Assertion (A): Generally the candle flame is yellow.
Reason (R): The flame of a candle is yellow due to the presence of unburnt
carbon particles.
Answer:
(a) Both A and R are true and R is the correct
explanation of A.
A candle flame is generally yellow due to the presence of
unburnt carbon particles when light falls on these particles, they scatter a
yellow colour. This shows, that the combustion of hydrocarbons in wax (candle)
is not complete.
Question 18.
Assertion: The probability of survival of an organism
produced through sexual reproduction is more than that of organism produced
through asexual mode.
Reason: Variations provide advantages to individuals
for survival. [1]
Solution:
(a) Both A and R are true, and R is the
correct explanation of A.
Explanation: The Probability of survival of an
organism produced through sexual reproduction is more than that of organism
produced through asexual mode. This is because an organism produced by sexual
reproduction has a greater survival rate as variations provide advantages to
individuals for survival and they can adapt to various environments. Thus, both
assertion and reason are true and reason is the correct explanation of
assertion.
Question 19.
Assertion (A): Ureter forms the common passage for both the
sperm and urine.
Reason (R): It never carries sperm.
Answer:
(d) A is
false, but R is true.
In males, the urethra forms the common passage for both
the sperms and urine, whereas ureters are tubes – that propel urine from the
kidneys to the urinary bladder. It never carries sperm.
Question 20.
Assertion: Biodegradable substances result in the formation
of compost and natural replenishment. Reason: It is due to the breakdown of
complex inorganic substances into simple organic substances. [1]
Solution:
(c) A is true but R is false.
Explanation: Biodegradable
substances such as food waste, humans and animal excreta, plant products, dried
leaves, grass, fruits, flowers, food wastes, wood and other remains of the death
of living creature result in the formation of compost and natural replenishment.
This is because complex organic substances like carbohydrates, proteins, and
lipids present in these substances are converted into simple inorganic
substances like hydrogen, oxygen, calcium, iron, and sodium. These inorganic
substances are released back into the soil and they serve as nutrients for the
growth of plants, thereby balancing the ecosystem. Thus, assertion is true but
reason is false.
Section B
Questions No. 21 to 26 are Very Short Answer Questions.
Question 21.
A metal forms two types of oxide and rust in moisture. Write
the formulas of oxides and name the metal. Also, give the name of the metal used
in the hot water apparatus.
Answer:
Iron forms two oxides iron (II) oxide
and iron (III) oxide, i.e. ferrous and ferric oxide respectively.
Formula of
ferrous oxide = FeO
Ferric oxide = Fe2O3
Copper is
used in hot water apparatus since it is a good conductor of heat. (2)
Question 22.
State the post-fertilisation changes that lead to fruit
formation in plants. [2]
Solution:
The post-fertilisation changes that
lead to fruit formation in plants are:
- Zygote divides several times to form an embryo within the ovule.
- The ovule develops a tough coat and is gradually converted into a seed.
- The ovary grows rapidly and ripens to form a fruit.
- The petals, sepals, stamens, style and stigma may shrivel and fall off.
Question 23.
State the functions of the following plant hormones.
(a)
Abscisic acid
(b) Cytokinin
Or
Mention the correct positions of the
pancreas, thyroid gland, pituitary gland, and adrenal gland in the human body.
Also, mention the hormones released by them.
Answer:
(a) Function of
Abscisic acid:
(i) It inhibits growth.
(ii) It causes dormancy of seeds,
wilting of leaves,
(iii) Abscisic acid leads to stomatal closure. (1)
(b)
Function of Cytokinin:
(i) Cytokinin promotes cell division and reduces
apical dominance.
(ii) It delays aging in leaves. (1)
Or
(a) The
pancreas is found just below the stomach. It secretes insulin.
(b) The
thyroid gland is found just below the neck and it secretes thyroxine.
(c) The
pituitary gland is the master gland. It is present at the base of the brain. It
growth hormone.
(d) Adrenal glands are present on top of kidneys it secrete
steroid hormones like aldosterone. (2)
Question 24.
The refractive indices of three media arc given below:
| Medium | Refractive Index |
| A | 1.6 |
| B | 1.8 |
| C | 1.5 |
A ray of light is travelling from A to B and another ray is travelling from B
to C.
(a) In which of the two cases the refracted ray bends towards the
normal?
(b) In which case does the speed of light increase in the second
medium?
Give reasons for your answer. [2]
Solution:
(a) When light
travels from an optically rarer medium to an optically denser medium it moves
towards the normal. Since nB > nA, hence the light ray
will bend towards the normal on passing from medium A to B.
(b) The speed of
the light will increase when the light travels from B to C, Since nC
< nB and υ =(c/n), the speed of light ray will increase in the
second medium i.e., medium C.
Question 25.
Beams of light are incident through holes A and B and emerge
out of box through the holes C and D respectively, as shown in the
figure.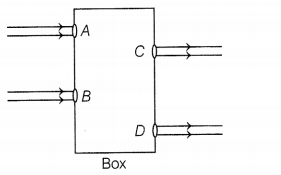
What could be inside the box?
Or
Two lamps, one rated
at 100W-220V and the other at 60W-220V are connected in parallel to the electric
mains supply. What current is drawn from the line, if the supply voltage is
220V?
Answer:
Here, the emergent rays are parallel to the direction of the
incident ray. Therefore, a rectangular glass slab could be inside the box as the
extent of bending of the light ray at the opposite parallel faces AB (air-glass
interface) and CD (glass-air interface) of the rectangular glass slab are equal
and opposite. This is why the ray emerges parallel to the incident ray. (2)
Or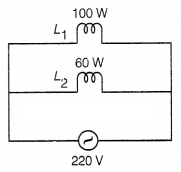
Given, potential, V = 220 V
Power, P1 = 100
W
Power, P2 = 60 W
∴ Current, I1 = \(\frac{P_1}{V}\)
= \(\frac{100}{220}\) = 0.45 A
Current, I2 = \(\frac{P_2}{V}\) =
\(\frac{60}{220}\) = 0.27 A
∴ Total current drawn, I = I1 +
I2
= 0.45 + 0.27
= 0.72 A (2)
Question 26.
Study the food chain given below and answer the questions
that follow: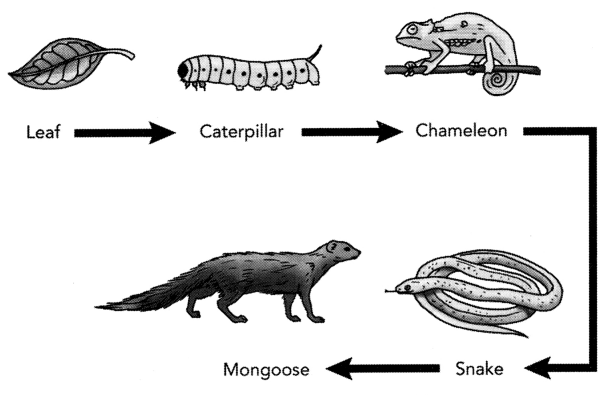
(a) If the amount of energy available at the third trophic
level is 100 joules, then how much energy will be available at the producer
level? Justify your answer.
(b) Is it possible to have 2 more trophic levels
in this food chain just before the fourth trophic level?
Justify your answer.
[2]
Solution:
(a) According to the 10 percent law, only 10% of the energy
is transferred to each trophic level starting from the first trophic level or
producer level. If the amount of energy available at the third trophic level is
100 joules, then only 1000 joules of energy will be available at the second
trophic level and 10000 Joules will be available at the producer level or first
trophic level.
(b) No, since the loss of energy at each step is so great that very little usable energy will remain after 4 trophic levels.
Section C
Questions No. 27 to 33 are Short Answer Questions.
Question 27.
Answer the following questions.
(a) State the functional
group present in alcohols.
(b) Give the general formula of alcohol.
(c)
What is meant by denatured alcohol?
Answer:
(a) -OH (1)
(b)
CnH2n+1OH (1)
(c) To prevent the misuse of alcohol
produced for industrial purposes, it is made unfit for drinking by adding
poisonous substances like methanol to it. This is called denatured alcohol.
(1)
Question 28.
An element ‘M’ with electronic configuration 2 8 3 combines
separately with Cl–, \(\mathrm{SO}_4^{-2}\) anions. Write the
chemical formulae of the compounds formed. Predict with the suitable reason the
nature of the bond formed by element ‘M’ in general. How will the electrical
conductivity of the compounds formed vary concerning ‘M’?
OR
A
reddish-brown metal ‘X’, when heated in air, gives a black compound ‘Y, which
when heated in presence of H2 gas gives ‘X’ back. ‘X’ is refined by the process
of electrolysis; this refined form of ‘X’ is used in electrical wiring.
Identify ‘X’ and ‘Y. Draw a well-labeled diagram to represent the process of
refining ‘X’. [3]
Solution:
The Chemical formulae of the compounds formed
will be MCl3 and M2(SO4 )3. The
nature of bond formed by element ‘M’ in general is Ionic bond because it can
acquire a stable electronic configuration of neon (2, 8) by losing its three
valence electrons to form M3+ cation. Compounds formed will conduct electricity
in liquid or molten state but not in solid state in contrast to ‘M’.
OR
(a) ‘X’ – Copper! Cu and ‘Y’ – CuO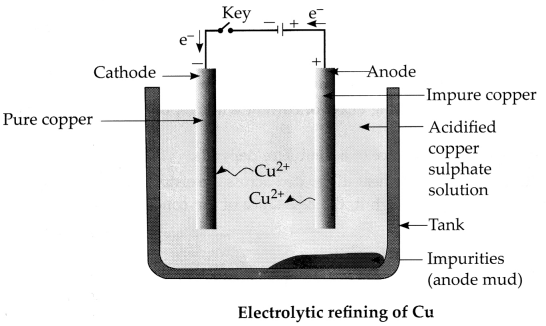
Question 29.
Why is the flow of signals in a synapse from the axonal end
of one neuron to the dendritic end of another neuron, but not the reverse?
Answer:
The synapse acts, like a one-way valve because the chemical substance
is present only on one side of the gap. This chemical diffuses towards the
dendrite end of the next neuron where it generates an electrical signal. Since
the chemicals are absent at the dendritic end of the neuron, the nerve impulse
can go across only from one side (which contains the chemical substance). In
this way, it is ensured that nerve impulses travel in only one direction
(through a particular set of neurons). (3)
Question 30.
What is the probability of a girl or a boy being bom in a
family? Justify your answer. [3]
Solution:
There are 50% chances that a
girl may be born and 50% chances that a boy may be born. It can be explained as
follows: Most human chromosomes have a maternal copy and a paternal copy. We
have 22 such chromosomes. One pair of chromosomes called sex chromosomes is odd
and is in not always being a perfect pair. Women have a perfect pair of sex
chromosomes, both called X, (XX). But men have a mismatched pair of sex
chromosomes in which one is normal-sized – X chromosome while the other is a
short one called Y chromosome (XY). A child receives one chromosome from mother
which is essentially X chromosome. A child who inherits an X chromosome from her
father will be a girl, and one who inherits a Y chromosome from him will be a
boy.
Question 31.
The figure shows three identical bulbs A, B, and C which are
connected to a battery of supply voltage V. When the switch S is closed, discuss
the change in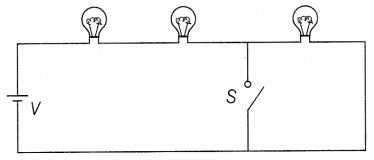
(a) the illumination of the three bulbs.
(b) the power
dissipated in the circuit.
Answer:
When the switch is open,
VA = VB = VC = \(\frac{V}{3}\)
and
PA = PB = PC = \(\frac{(V /
3)^2}{R}=\frac{V^2}{9 R}\) = P (say)
(a) When the switch closed, then bulb C
is short-circuited, and will be no current through C, PC = 0
VA = VB = \(\frac{V}{2}\)
⇒ PA =
PB = \(\frac{(N / 2)^2}{4 R}=\frac{9}{4} P\) (1\(\frac{1}{2}\))
(b) Power dissipated, P1 = PA + PB +
PC = 3P
Thus, Pt = PA + PB +
PC
= \(\frac{9}{4} P+\frac{9}{4} P\) + 0
= \(\frac{9}{2}\)P
= \(\frac{3}{2}\)P1 (1\(\frac{1}{2}\))
Question 32.
(i) State the law that explains the heating effect of current
concerning the measurable properties in an electrical circuit.
(ii) List the
factors on which the resistance of a conductor depends. [2 + 1]
Solution:
(i) Joule’s law of heating states that the heat dissipated across a resistor is
directly proportional to the square of the current flowing through it, the
resistance of the conductor and duration of flow of current.
H =
I2Rt
Where H = Heat dissipated across resistor
I = Current
R
= Resistance of the conductor t = time or duration of flow of current
(ii) The resistance of a conductor depends on:
(a) Length of the
conductor
(b) Area of the cross-section
(c) Nature of material
(d)
Temperature of the conductor.
Question 33.
How do Mendel’s experiments show that traits may be dominant
or recessive?
Answer:
Mendel crossed a pure tall pea plant (TT) with a
pure dwarf pea plant (tt) and observed that all the progeny were hybrid tall
(Tt), i.e. only one of the traits was able to express itself in the
F1 generation, which is the dominant trait. The other trait is called
the recessive trait which remains suppressed.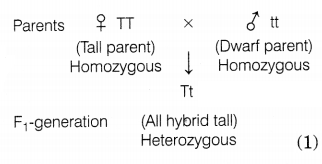
However, when he self-crossed the plants of F1
generation, he observed that one-fourth of the plants were dwarf and
three-fourths were tall.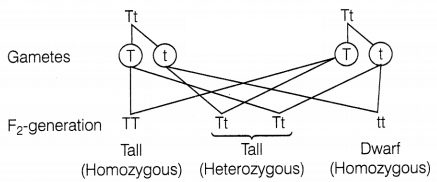
The expressed trait T for tallness is dominant, while the
trait ‘t’ of dwarfness is recessive. Thus, Mendel’s experiments show that traits
may be dominant or recessive. (2)
Section D
Questions No. 34 to 36 are Long Answer Questions.
Question 34.
(a) Rehmat classified the reaction between Methane and
Chlorine in the presence of sunlight as a substitution reaction. Support
Rehmat’s view with suitable justification and illustrate the reaction with the
help of a balanced chemical equation.
(b) Chlorine gas was prepared using
electrolysis of brine solution. Write the chemical equation to represent the
change. Identify the other products formed in the process and give one
application of each.
OR
Raina while doing certain reactions observed that
heating of substance ‘X’ with vinegar like smell with a substance ‘Y (which is
used as an industrial solvent) in presence of cone. Sulphuric acid on a water
bath gives a sweet-smelling liquid ‘Z’ having molecular formula
C4H8O2. When heated with caustic soda (NaOH),
‘Z’ gives back the sodium salt of and the compound ‘Y.
Identify ‘X’, ‘Y, and
‘Z’. Illustrate the changes with the help of suitable chemical equations.
[5]
Solution:
(a) Rehmat’s observation is correct as the hydrogen atoms
are substituted by hetero atom i.e., Cl Reaction between methane and chlorine in
presence of sunlight can be repersented as: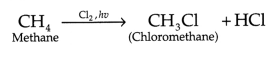
In this reaction, the chlorine free radial, abstracts
hydrogen from methyl group which then riacts
with Cl2 to form
chioromethane, It is can be represented as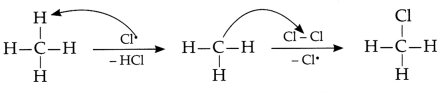
In the above reaction, the hydrogen atoms are substituted by
chlorine atom and hence this reaction is considered as substitution
reaction.
(b) When electricity is passed through a concentrated solution of NaCl, also
called as Brine, it decomposes and results in the formation of Sodium Hydroxide
(NaOH), Chlorine gas (Cl2), and Hydrogen gas (H2). The
chemical equation can be represented as:
2NaCl(aq) + 2H2O(l)
2NaOH(aq) + Cl2(g) + H2(g)
Apart from chlorine gas
(Cl2), the other products formed in the reaction are sodium hydroxide
or caustic soda (NaOH) and hydrogen gas (H2).
Uses
Sodium
hydroxide (NaOH) or Caustic soda is used in the preparation of soaps and
detergents
Hydrogen gas (H2) is used in the manufacture of ammonia
for fertilizers.
OR
When ethanoic acid with vinegar like smell is heated
with ethanol(which is used as an industrial solvent) in the presence of cone,
sulphuric acid on a water bath, a sweet-smelling liquid called ethyl ethanoate
is formed. The molecular formula of ethyl ethanoate is
C4H8O2. The chemical equation can be
represented as: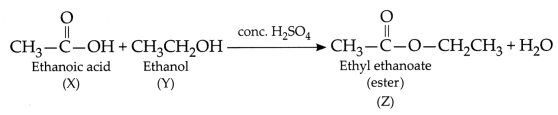
Therefore, the substances,
X – Ethanoic acid/ acetic acid/
CH3COOH
Y – Ethanol/ Ethyl alcohol/
C2H5OH
Z – Ethyl ethanoate/ Ester –
CH3COOC2H5
When ethyl ethanoate (Z) is
heated with a dilute solution of caustic soda or sodium hydroxide (Na), it gives
back the original ethyl alcohol and sodium salt of the original carboxylic acid.
Such a reaction is known as a saponification reaction and it can be represented
as: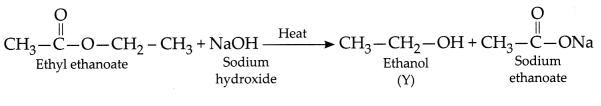
Question 35.
(a) Explain the difference between androecium and
gynoecium.
(b) How does reproduction help in providing stability to the
population of a species?
Or
(a) What is cellular respiration? How many ATP
molecules are obtained by the oxidation of one glucose atom?
(b) With the
help of an experiment, show that chlorophyll is necessary for
photosynthesis.
Answer:
(a) Differences between androecium and gynoecium
are as follows:
| Androecium | Gynoecium |
| This is the male reproductive organ of a plant. | This is the female reproductive organ of a plant. |
| Each unit of this is called a stamen. | Each unit of this is called a carpel/pistil. |
| The terminal bloated part of the stamen is called the anther, in which male gametes or pollen grains are produced. | The lower bloated part of the carpel/pistil is called an ovary, in which the ovule is present, A Female gamete or egg is produced in the ovule. |
(b) A species occupies a well-defined niche in an ecosystem, using its
ability to reproduce. During reproduction, copies of DNA pass from one
generation to the next. This copying of DNA takes place with consistency in
reproducing organisms and This is important for the maintenance of body design
features (physiological as well as structural) which allows the organism to use
that particular niche. Reproduction is, therefore, linked to the stability of
the population of a species. (2\(\frac{1}{2}\))
Or
(a) In cells, the
biochemical process in which glucose is oxidized in the presence of oxygen is
known as aerobic or cellular respiration. During the oxidation of food, energy
gets released which is stored in the form of ATP through the electron transport
system. On complete oxidation, one molecule of glucose forms 38 ATP molecules.
(2)
(b) Experiment to show that chlorophyll is necessary for photosynthesis: In
this experiment plants having variegated leaves are selected. These plants are
kept in the dark for 48 hours to make them starch-free. Then, the paint is
researched by keeping it in the sun. After a few hours, the green and non-green
areas are marked on a leaf. Following this, the leaf is tested for starch in
which only green areas i.e. chlorophyll containing part turn blue-black due to
the presence of starch. This shows that chlorophyll is essential for
photosynthesis.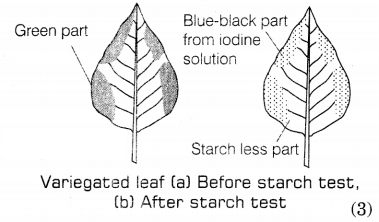
Question 36.
The above image shows a thin lens of focal length 5m. [1 + 2
+ 2]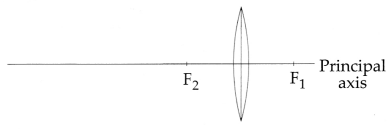
(i) What is the kind of lens shown in the above figure?
(ii) If a real inverted image is to be formed by this lens at a distance of 7m
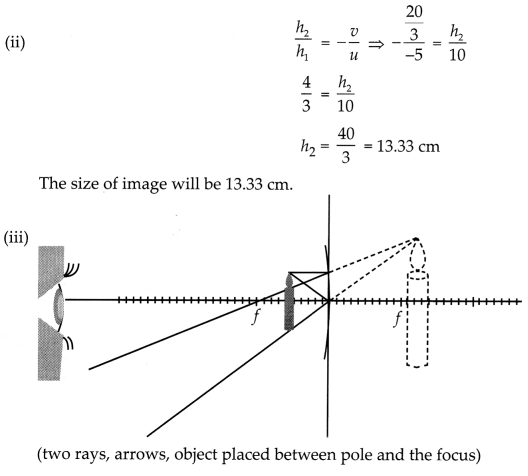
from the pole, then show with calculation where should the object be placed?
(iii) Draw a neatly labelled diagram of the image formation mentioned in
(ii)
OR
A 10 cm long pencil is placed 5 cm in front of a concave mirror
having a radius of curvature of 40 cm. [2 + 1 + 2]
(i) Determine the position
of the image formed by this mirror.
(ii) What is the size of the image?
(iii) Draw a ray diagram to show the formation of the image as mentioned in the
part (i).
Solution:
(i) Convex Lens
(ii) Given, υ = 7 m, f = 5 m
We
know that, \(\frac{1}{f}=\frac{1}{υ}-\frac{1}{u}\)
Putting the values in the
equation, we get,
\(\frac{1}{5}=\frac{1}{7}-\frac{1}{u}\)
\(\frac{1}{u}=\frac{1}{7}-\frac{1}{5}=\frac{5-7}{35}=\frac{-2}{35}\)
u = –
\(\frac{35}{2}\) = -17.5 m
Hence, the object will be placed 17.5 m on the
left of the convex lens.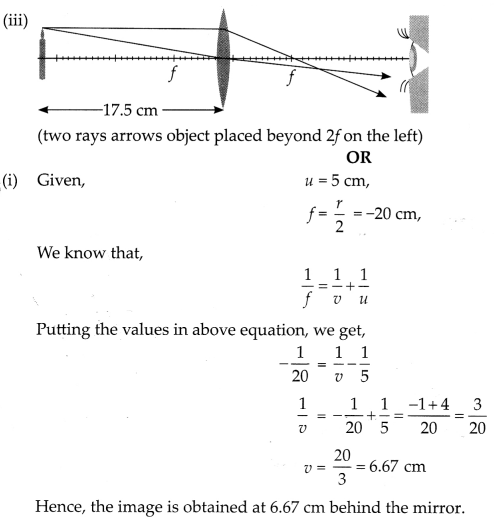
Section E
Questions No. 37 to 39 are case-based/data-based questions with 2 to 3 short sub-parts. Internal choice is provided in one of these sub-parts.
Question 37.
Four groups of students were assigned separately to the
experiment on the interaction of iron nails with a solution of copper sulphate.
Each group recorded the observations as given below in the table.
| Group of Students | Initial Colour of Solution | Final Colour of Solution | Change in the Iron Nail |
| A | Blue | Colourless | Brown Coat |
| B | Green | Green | Brown Coat |
| C | Blue | Blue | Brown Coat |
| D | Blue | Light Green | Brown Coat |
(a) Which type of reaction is observed in the given experiment?
(b) Which
group of students recorded all the observations correctly? Also, write the
chemical equation involved.
Or
Discuss the reason behind the brown coating
on iron nails.
Answer:
(a) The change in colour of the initial and final
solution and the deposition of a layer on an element indicates the occurrence of
displacement reaction. (2)
(b) The blue colour of copper sulphate changes into a light green colour
solution due to the formation of ferrous sulphate by a displacement
reaction.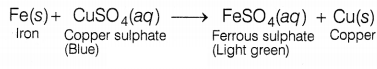
The displaced copper gets deposited on the iron nail and
forms a brown coating on it. Hence, students of the D group recorded all the
correct observations. (2)
Or
The brown coating on iron nails is due to
copper deposition. According to the reactivity series, iron is more reactive
than copper, so, it will displace copper from its sulphate solution, and then
solid copper will get deposited on the iron nail. (2)
Question 38.
Figures (a) to (d) given below represent the type of ear
lobes present in a family consisting of 2 children – Rahul, Nisha and their
parents. [4]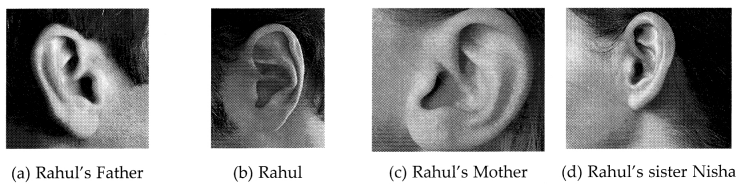
Excited by his observation of different types of ear lobes
present in his family, Rahul conducted a survey of the type of ear lobes found
{Figure (e) and (f)} in his classmates. He found two types of ear lobes in his
classmates as per the frequency given below:
| Sex | Free | Attached |
| Male | 36 | 14 |
| Female | 31 | 19 |
Based on the above data answer the following questions.
(a) Which of the
two characteristics – ‘free ear lobe’ or ‘attached ear lobe’ appears to be
dominant in this case? Why?
(b) Is the inheritance of the free ear lobe
linked with sex of the individual? Give reason for your answer.
(c) What type
of ear lobe is present in father, mother, Rahul and his sister Nisha? Write the
genetic constitution of each of these family members which explains the
inheritance of this character in this family?
(Gene for Free ear lobe is
represented by F and gene for attached ear lobe is represented by f for writing
the genetic constitution).
OR
Suresh’s parents have attached earl obes.
What type of ear lobe can be seen in Suresh and his sister , ‘ Siya? Explain by
giving the genetic composition of all.
Solution:
(a) Free ear lobe is
dominant because it is found in a large majority of the population.
(b) No, it is not sex-linked. As per the data of the family as well as the class, it is indicated that free ear lobe is present in males as well as in females.
(c) Father – Ff (free ear lobe), Mother – Ff (free ear lobe), Rahul – ff
(attached ear lobe) and Nisha – Ff (free ear lobe).
OR
Both Suresh’s
father and mother have attached ear lobes (ff). If both parents have recessive
character, then all the children will have recessive character only. Therefore,
Suresh and her sister siya will also have an attached ear lobe (ff).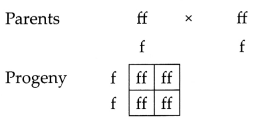
Suresh’s Father ff (attached ear lobe),
Suresh’s Mother ff
(attached ear lobe),
Suresh – ff (attached ear lobe),
Siya – ff (attached
ear lobe).
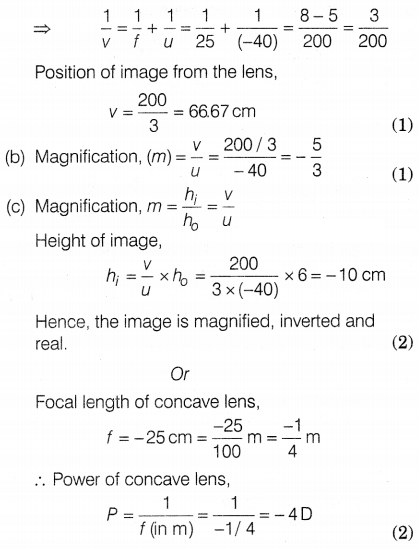
Question 39.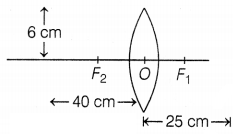
The above images show the position and height of an object in
front of a convex lens. A 6 cm tall object is placed perpendicular to the
principal axis of a convex lens of focal length 25 cm. The distance of the
object from the lens is 40 cm.
(a) Based on the text and data in the above
paragraph, determine the position of the image.
(b) The magnification
produced by the convex lens.
(c) Size and nature of the image formed.
Or
Find the power of the lens.
Answer:
(a) Given, the height of the
object, h0 = 6 cm
Focal length, f = 25 cm
Distance of object, u
= -40 cm
Using lens formula, \(\frac{1}{f}=\frac{1}{v}-\frac{1}{u}\)