CBSE Sample Papers for Class 10 Science Set-11
Class 10thCBSE Sample Papers for Class 10 Science Set-11
CBSE Sample Papers for Class 10 Science Set 11
Time: 3 Hours
Maximum Marks: 80
Instructions
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
- Section A consists of 20 objective-type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
- Section D consists of 3 Long Answer type questions carrying 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 4 marks each with sub-parts.
Section A
Select and write the most appropriate option out of the four options given for each of the questions 1-20.
Question 1.
Consider the given flowchart.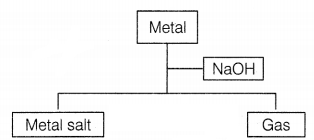
Which of the following two combinations are correct?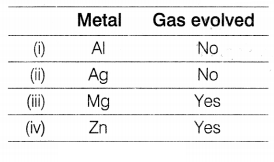
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and
(iv)
(d) (i) and (iv)
Answer:
(c) (ii) and (iv)
The correct
combination is (ii) and (iv) as [1]
(i) When Al reacts with NaOH, the salt of
aluminium is formed with the evolution of hydrogen gas.
2Al (s) + 6NaOH (aq)
→ 2Na3AlO3 + 3H2 ↑
(ii) When silver and
sodium hydroxide react together, no reaction occurs. Hence no evolution of gas
occurs.
(iii) Magnesium and sodium hydroxide do not react with each other.
Hence, no evolution of gas.
(iv) When zinc reacts with sodium hydroxide, zinc
salt is formed by the evolution of hydrogen gas.
Zn (s) + 2NaOH (aq) →
Na2ZnO2 + H2 ↑
Question 2.
Dominant alleles are expressed exclusively in a heterozygote,
while recessive traits are expressed only if the organism is ……………… for the
recessive allele.
(a) Homozygous
(b) Heterozygous
(c) Normal
(d)
None of these
Answer:
(a) Homozygous
Explanation: According to Mendel’s
law of dominance, when there is a heterozygote (an organism with two different
alleles for a trait), one allele will dominate and mask the presence of the
other allele. Only the dominant allele will be expressed and contribute to the
physical characteristics (phenotype). The recessive allele remains hidden but
can still be passed on to offspring in the same way as the dominant allele. The
recessive trait will only be visible in offspring who inherit two copies of this
recessive allele.
Question 3.
__________ can be used as an acid-base indicator by a visually
impaired student.
(a) Litmus
(b) Vanilla essence
(c) Turmeric
(d)
Petunia leaves
Answer:
(b) Vanilla essence
Vanilla essence is an
olfactory indicator. So, its smell is different in acidic and basic media which
can be detected easily by a visually impaired student. It has a characteristic
pleasant smell. If a basic solution like sodium hydroxide solution is added to
it, an acidic solution like hydrochloric acid, however, does not destroy the
smell of Vanilla extract.
Question 4.
Which of the following salts does not contain water of
crystallization?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer:
(b) Baking soda
Explanation:
Baking soda: It is
sodium bicarbonate (NaHCO3) in anhydrous form without any water of
crystallisation.
Blue Vitriol: It is hydrated salt of copper sulphate containing 5 molecules of water of crystallisation (CuSO4. 5H2O).
Washing soda: It is hydrated salt of sodium carbonate containing 10 molecules of water of crystallisation (Na2CO3.10H2O)
Gypsum: It is hydrated salt of calcium sulphate containing 2 molecules of water of crystallisation (CaSO4.2H2O
Question 5.
The reaction between potassium bromide and silver nitrate is
an example of
(a) combination reaction
(b) decomposition reaction
(c)
double displacement reaction
(d) displacement reaction
Answer:
(c)
double displacement reaction
The reaction between potassium bromide and
silver nitrate forms two products: silver bromide and potassium nitrate.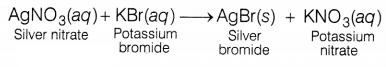
This reaction is an example of a double displacement reaction
as Ag and K are displaced by each other.
Question 6.
A farmer wants to grow banana plants genetically similar
enough to the plants already available in his field. Which one of the following
methods would you suggest for this purpose?
(a) Regeneration
(b)
Budding
(c) Vegetative propagation
(d) Sexual reproduction
Answer:
(c) Vegetative propagation
Explanation: Vegetative propagation is a method of
plant reproduction that involves using vegetative parts of a plant, such as
stems, roots, or leaves, to create new individuals that are genetically
identical to the parent plant. This method ensures that the desired
characteristics of the parent plant, including fruit quality and traits, are
preserved in the new plants. This method avoids the genetic variation that can
occur through sexual reproduction, which involves the combination of genetic
material from two different parent plants and may result in offspring with
different traits.
Question 7.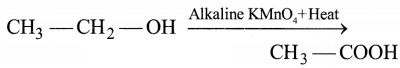
In the above-given reaction, alkaline KMnO4 acts
as
(a) reducing agent
(b) oxidizing agent
(c) catalyst
(d)
dehydrating agent
Answer:
(b) oxidizing agent
KMnO4 acts as
an oxidizing agent because it removes hydrogen from
CH3CH2OH and adds one oxygen to it.
Question 8.
Characters transmitted from parents to offspring are present
in:
(a) Cytoplasm
(b) ribosome
(c) Golgi bodies
(d) genes
Answer:
(d) genes
Explanation: Characters are transmitted from parents to
offspring through genes. Genes are the heredity units of the body in living
organisms. Chromosomes in the nucleus of a cell contain information for the
inheritance of features from parents in the form of DNA (Deoxyribonucleic acid).
This DNA contains genes.
Question 9.
The graph shows, how the amount of carbon dioxide taken by a
plant.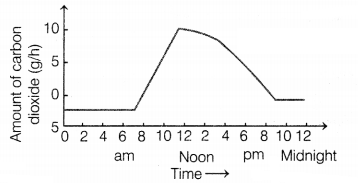
Why is the uptake of CO2 higher during day
time?
(a) due to the large number of structures in the daytime
(b) due to
photosynthesis
(c) due to transpiration
(d) due to more water
absorption
Answer:
(b) due to photosynthesis
Plants take in
CO2 from the atmosphere for preparing food. At night or early
morning, photosynthesis does not occur hence, they take in oxygen and give out
CO2.
Question 10.
Which of the following statements is not true about
Thyroxine?
(a) Thyroxine regulates the basal metabolism of our body.
(b)
Iodine is an important component required for the synthesis of Thyroxine.
(c)
Under secretion of Thyroxine causes simple goiter.
(d) Iron is essential for
the synthesis of Thyroxine.
Answer:
(d) Iron is essential for the
synthesis of Thyroxine.
Explanation: Iodine, not iron, is required to
synthesize Thyroxine. It controls the glucose, protein, and fat metabolism of
the body. The thyroid gland produces Thyroxine, which is also known as thyroid
hormone.
Question 11.
Which part of the human brain controls body temperature?
(a) Pituitary
(b) Diencephalon
(c) Hypothalamus
(d) None of the
above
Answer:
(c) Hypothalamus
The hypothalamus controls and regulates
the temperature of the body, the urge to eat, drink, sleep, etc.
Question 12.
In which direction do afferent neurons carry nerve
impulses?
(a) From the central nervous system (CNS) to muscles
(b) From
the CNS to receptors
(c) From receptors to the CNS
(d) From effector
organs to the CNS
Ans.
(c) From receptors to the CNS
Explanation:
Afferent neurons, also known as sensory neurons, are responsible for
transmitting nerve impulses from sensory receptors in the peripheral nervous
system to the central nervous system (CNS). These neurons carry sensory
information, such as touch, temperature, pain, and other stimuli, from various
parts of the body to the CNS for processing and interpretation.
Question 13.
Four students set up the circuit for the experiment of Ohm’s
law as shown. Which of the following options is correct?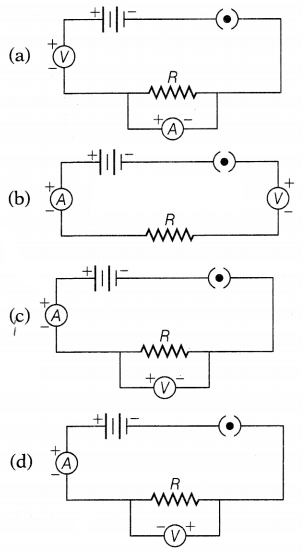
Answer:
In the circuit, the ammeter should always be connected in
series and the voltmeter should always be connected in parallel combination.
Hence, the correct circuit set-up is shown below.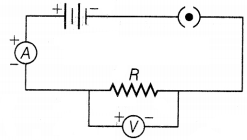
Question 14.
A current flows in a wire, running between the S and N poles
of a magnet lying horizontally, as shown inithe figure below: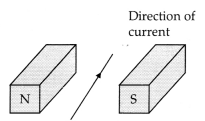
The force on the wire due to the magnet is directed.
(a)
From N to S (c)
(b) From S to N (d)
(c) Vertically downwards
(d)
Vertically upwards
Answer:
(c) Vertically downwards
Explanation: Force
on the conductor is calculated using Fleming’s left-hand rule.
Question 15.
A food web is the
(a) food that a spider collects using
its web
(b) network of interlinked trophic levels
(c) network of
interlinked food chains
(d) display of food items on a website
Answer:
(c) network of interlinked food chains
A food web is a network of interlinked
food chains operating at various trophic levels.
Question 16.
Mutual induction is a process in which current is induced in
the neighboring coil if current flows in a coil. In the figure shown
below: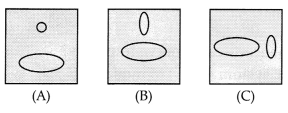
(a) Maximum in the situation (A)
(b) Maximum in situation
(B)
(c) Maximum in the situation (C)
(d) Same in all situations
Answer:
(a) Maximum in situation (A)
Explanation: As both the coils are in
the same plane the induced current is found to be highest when 1 the direction
of the coil is at the right angle to the magnetic field.
Directions (Q.Nos. 17-20) consist of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both
A and R are true, but R is not the correct explanation of A.
(c) A is true,
but R is false.
(d) A is false, but R is true.
Question 17.
Assertion (A): Diamond does not conduct electricity.
Reason (R): Diamond has a high refractive index.
Answer:
(b) Both A and R
are true but R is not the correct explanation of A.
The correct reason is,
that due to the absence of free electrons, diamond does not conduct
electricity.
Question 18.
Assertion: Electric current flowing through a metallic wire
is directly proportional to the potential difference across its ends.
Reason:
Ohm’s law expression V = IR, where R (resistance) of the wire is always
varying.
Answer:
(c) A is true, but R is false.
Explanation: Ohm’s law
states that the electric current flowing through a metallic wire is directly
proportional to the potential difference across its two ends. The expression is
written as :
V = IR
Here, R (resistance of the wire) is a constant value,
so only the statement will be valid.
V ∝ I only if \(\frac{V}{I}\) =
constant
Question 19.
Assertion (A): DNA copying is necessary during
reproduction.
Reason (R): DNA copying leads to the transmission of characters
from parents to offspring.
Answer:
(a) Both A and R are true and R is the
correct explanation of A.
DNA copying is necessary during reproduction
because it leads to the transmission of characters from parents to offspring and
brings about variation.
Question 20.
Assertion: Hydrogen peroxide is kept in coloured bottles.
Reason: Hydrogen peroxide is a moderately reactive metal that can react with
light or heat slowly to produce water.
Answer:
(c) A is true but R is
false
Explanation: Hydrogen peroxide is a highly reactive metal that can
react with light or heat to produce water. It decomposes into water and oxygen
in the presence of sunlight. To prevent this reaction with light and heat it is
stored in coloured bottles so that light cannot pass through it.
Section B
Questions No. 21 to 26 are Very Short Answer Questions.
Question 21.
What is a covalent bond? What type of bond exists in
CCl4 and CaCl2?
Answer:
The chemical bonds formed
between two atoms by the sharing of electrons between them is known as a
covalent bond. (1)
- CCl4 – Covalent bond
- CaCl2 – Ionic bond (1)
Question 22.
Answer the following questions:
(i) Define the power of a
lens.
(ii) Name the lens that has:
(a) Negative power.
(b) Positive
power.
Answer:
(i) The ability of a lens to converge the rays of light
falling on it is called the power of the lens,
(ii) (a) The focal length of a
concave lens is negative, so its power is negative.
(b) The focal length of a
convex lens is positive, so its power is positive.
Question 23.
Goitre is caused by which endocrine gland? Write one primary
prevention.
Or
Explain the functions of a neuron.
Answer:
Goitre is
caused by the deficiency of thyroxine hormone secreted by the thyroid gland.
Uptaking an optimum amount of iodine helps in preventing this disease. In hilly
areas, iodine is deficient in water which causes goitre disease in most of the
population in that area. Iodized salt uptake can help in the prevention of this
disease. (2)
Or
Functions of a neuron The neuron receives information from
receptors as electrical impulses, at its dendritic end. The impulse then travels
from the dendrite to the cell body and further along the axon to its end. At the
end of the axon, an electrical impulse leads to the release of some chemicals.
These chemicals cross the synapse and reach the next neuron. This is how nerve
impulses travel through the body. Thus, neurons are important in receiving
information from the surroundings and in sending it to the effector. (2)
Question 24.
The diagram shows one layer of carbon atoms in
graphite.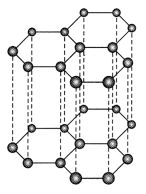
(i) Identify the type of bonding in graphite.
(ii) Which
property of graphite makes it suitable for use as a dry lubricant? Explain.
Answer:
(i) Between the carbon atoms in each layer covalent bond is found and
Van Der Waal’s forces are found between the layers of carbon atoms in
graphite.
(ii) Graphite is soft which makes it suitable for use as a dry lubricant. Between the carbon atoms in each layer covalent bond is found and weak Van Der Waals forces are found between the layers of carbon atoms in graphite. So, the layers can slide over one another making it a relatively soft substance.
Question 25.
What are magnetic field lines? Justify the following
statements.
(a) Two magnetic field lines never intersect each other.
(b)
Magnetic field lines are closed curves.
Or
You have two electric lamps
having ratings 40 W, 220 V and 60 W, 220 V. Which of the two has a higher
resistance? Give a reason for your answer. If these two lamps are connected to a
source of 220 V, which will glow brighter?
Answer:
The imaginary lines
representing the magnetic field around a magnet are known as magnetic field
lines.
(a) If two field lines intersect each other, this would mean that at
the point of intersection, the direction of the magnetic field is in two
directions, which is not possible. (1)
(b) The direction of field lines
outside a magnet is from the North pole to the South pole while it is from the
South to the North pole inside the magnet and thus forms closed curves. (1)
Or
We know that, power, P = \(\frac{V^2}{R}\)
Thus, resistance is
inversely proportional to power, i.e., higher power less will be resistance and
vice-versa. So, the electric lamp with a power rating of 40 W will have a higher
resistance as compared to a 60 W lamp. Lamps with higher power will glow
brighter. Hence, a lamp with 60 W power will glow brighter.
Question 26.
State the various characteristics of chemical reactions.
Answer:
Some of the characteristics of chemical reactions are:
- Formation of a precipitate
- Change in colour
- Change in temperature
- Change in the state.
- Evolution of a gas
Section C
Questions No. 27 to 33 are Short Answer Questions.
Question 27.
Explain the reactions of different metals with hot water,
cold water, and steam. Give one example with a properly balanced chemical
equation. Name of the two metals which do not react with any form of water.
Or
How is the method of extraction of metals high up in the reactivity series
different from that for metals in the middle? Why can be same process not be
applied for them? Name the process used for the extraction of these metals.
Answer:
Reaction of metal with water
(a) With cold water
(b) With hot water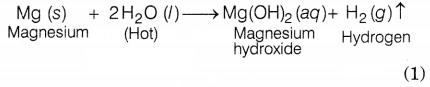
(c) With steam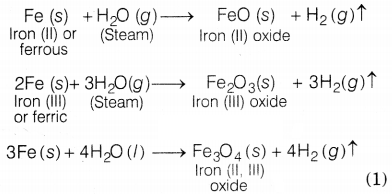
Gold and silver do not react with any form of water.
Or
The metals in the middle of the reactivity series (such as iron, zinc,
lead, copper, etc.) are moderately reactive. Thus, to obtain such metals from
their compounds, their sulphides and carbonates are first converted into their
oxides by the process of roasting and calcination respectively and then the
metal oxides are reduced to corresponding metal by using suitable reducing
agents such as carbon. (2)
On the other hand, metals which are high up in the
reactivity series (such as sodium, magnesium, calcium, aluminium, etc.) are very
reactive and cannot be obtained from their compound by heating with carbon.
Therefore, such metals are obtained by electrolytic reduction of their molten
salt. (1)
Question 28.
Obtain an expression for the magnification of an image formed
by a concave mirror.
Answer:
Consider the formation of the image A’B’ of
an object AB by a concave mirror. As shown in given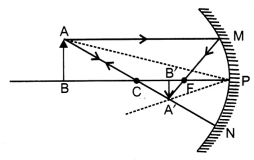
\(\frac{\mathrm{A}^{\prime}
\mathrm{B}^{\prime}}{\mathrm{AB}}=\frac{\mathrm{PB}^{\prime}}{\mathrm{PB}}\)
As per sign convention followed, PB = – u, PB’ = – υ, AB = size of the object =
+ h and A’B’ = size of the
image – h’.
Hence, we have
\(\frac{-h^{\prime}}{h}=\frac{-v}{-u}\)
or
\(\frac{h^{\prime}}{h}=\frac{-v}{u}\)
Thus, by definition of magnification of
the image, we have
Magnification, m =
\(\frac{h^{\prime}}{h}=-\frac{v}{u}\).
Question 29.
Explain how voluntary actions and reflex actions are
different from each other.
Answer:
The difference between involuntary
actions and reflex actions are as follows:
| Involuntary Actions | Reflex Actions |
| They involve the autonomic nervous system. | They involve all parts of the voluntary nervous system though they are not under our control. |
| They involve the functioning of the internal body parts. | They are concerned with emergencies. |
| The nervous system controlling involuntary actions has two divisions, sympathetic and parasympathetic. | There are no such divisions. |
| They occur in response to internal stimuli. | They commonly operate against harmful stimuli, which are generally external. |
| Most of the involuntary functions occur all the time. | Reflex actions occur occasionally. |
| Sometimes gap occurs between stimulus and response. | They are almost instant. |
| They are carried out by smooth muscles. | They are performed by striated muscles. |
| The beating of the heart and peristalsis. | Closing of eyes when light is flashed on them. |
Question 30.
How does Mendel’s experiment show that traits are inherited
independently?
OR
The given box diagram represents the ratio of females to
males or the sex ratio in our country for 10 decades (1901 to 2001). Answer the
following questions in light of your knowledge of sex determination and the data
presented in the box diagram.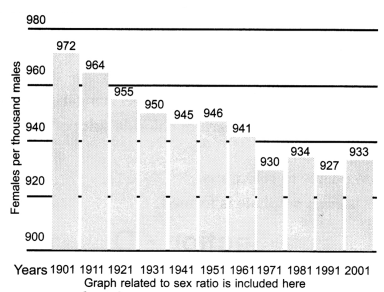
(i) What does the bar diagram show?
(ii) As per scientific
knowledge regarding sex determination, what should be the sex ratio or the
male-to-female ratio at a given point of time?
(iii) Assign one reason for
the trend showing deviation from the expected sex ratio.
(iv) Suggest a way
for which such a trend can be stopped.
Answer:
Mendel’s experiment show
that:
(i) When a cross was made between a tall pea plant with round seeds and
a short pea plant wrinkled seeds, the F1 progeny plants are all tall
with round seeds. This indicates that tall and round seeds are the dominant
traits.
(ii) When the F1 plants are self-pollinated and the F2 progeny show that some progencies tall with round seeds and some progenies were short plants with wrinkled seeds, which are the traits visible in the F2 generation.
(iii) There were also some progenies with new combinations like tall plants
with wrinkled seed short plants with round seeds.
OR
(i) Bar diagram shows
the proportion of females in the population over a decade.
(ii) 1 : 1 should
be the sex ratio or the male-to-female ratio at a given point of time.
(iii)
Female foeticide is the main reason for this trend showing deviation from the
expected sex ratio.
(iv) Banning sex tests of unborn babies; increasing
awareness and education will help to stop such trends.
Question 31.
Two resistors with resistances 5 Ω and 10 Ω respectively, are
to be connected to a battery of emf 6 V. How will you connect the resistances to
obtain
(a) maximum current?
(b) Calculate the strength of the total
current in the circuit in the two cases.
Answer:
(a) For obtaining maximum
current, the two resistors should be connected in parallel. (1)
(b) ∴ Total
current in the circuit, (parallel combination)
I = \(\frac{V}{R}=\frac{6
\times 3}{10}\) = 1.8 A
∴ Total current in the circuit, (series
combination)
I = \(\frac{V}{R}=\frac{6}{15}\) = 0.4 A (2)
Question 32.
A student wants to get the image of a candle flame on the
walls of a school laboratory by using a lens.
(a) Which type of lens should
he use and why?
(b) At what distance in terms of focal length F of the lens
should he place the candle flame, to get a magnified and diminished image
respectively, on the wall?
Answer:
(a) He should use a convex lens as real
images are formed by it. (1)
(b) For a magnified image, he should place the
candle flame between the focus (F) and centre of curvature (2F) of the lens. To
get a diminished image, he should place the candle flame beyond centre of
curvature (2F) of the lens. (2)
Question 33.
Give the basic features of the mechanism of inheritance.
Answer:
The basic features of inheritance are as follows:
- Characters are controlled by genes.
- Each gene controls one character.
- There may be two or more forms of gene.
- One form may be dominant over the other.
- Genes are present on chromosomes.
- An individual has two forms of gene whether similar or dissimilar. (\(\frac{1}{2}\) × 6)
Section D
Questions No. 34 to 36 are Long Answer Questions.
Question 34.
Explain the nutrition process in Amoeba.
OR
(i) Explain
the statement “Bile does not contain any enzyme but it is essential for
digestion”.
(ii) Explain the process of breathing in human beings.
Answer:
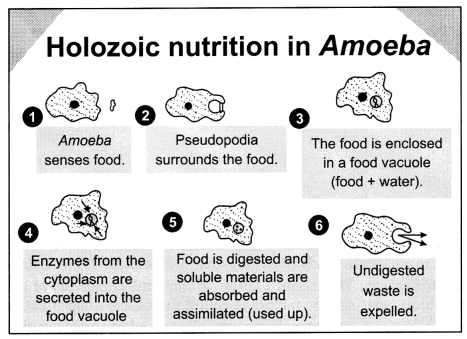
Amoeba follows holozoic nutrition.
It involves the following
steps:
- Ingestion – Amoeba engulfs the food by using its temporary finger-like projections called pseudopodia. This process is called ingestion.
- Digestion – The food which is taken inside the amoeba forms a food vacuole. Many enzymes are secreted into the food vacuole and the complex food molecule is converted into a simple and diffusible form.
- Absorption and assimilation – The digested food is absorbed by the cell by the diffusion process.
- Egestion – The undigested residue which remains in the vacuole is expelled.
OR
(i) Though bile juice secreted by liver has no enzymes
in it is still essential for digestion because:
- It makes the medium alkaline in the small intestine so that intestinal juice can perform its functions because the food from stomach which enters the small intestine is acidic in nature.
- It emulsifies fats so that enzymes like lipase can act upon fats to convert them into fatty acids and glycerol.
(ii) Breathing process occurs by inhalation and exhalation.
1. Inhalation:
It is the process by which oxygen is taken in through nostrils. During
inhalation process, the ribs move upwards and outwards due to contraction of
intercostal muscles. The diaphragm is lowered so that the volume of thoracic
cavity increases. As a result air is forced inside the lungs through
nostrils.
2. Exhalation: It is the process by which carbon dioxide is exhaled out from
lungs through nostrils. During this process the ribs moves inwards and diaphragm
comes back to its original position. The volume of thoracic cavity decreases so
air is forced out through the lungs.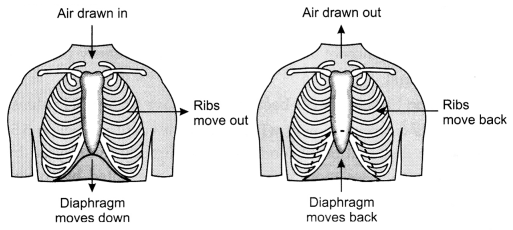
Question 35.
(a) Marriage between cousins is a taboo in most of the
countries of the world except in some Asian countries. Why should marriage
between close cousins be prevented and which measures will you take to prevent
such practices?
(b) Describe the process of fertilization in a flower.
Or
(a) Explain the events of double circulation.
(b) Differentiate between
fermentation in yeast and aerobic respiration based on the end products
formed.
Answer:
(a) Marriage between close cousins should be prevented as
the recessive traits with the genetic defects present in such families will
become homozygous and cause harmful diseases Children should be educated about
the ill effects so that they come to know about the defects in marriages among
close cousins. These are the measures that can be taken to prevent such
practices. (2)
(b) It is the process of fusion of male germ cells with the female gametes.
It gives rise to a zygote. As soon as the pollen lands on a suitable stigma, it
reaches the female germ cells in the ovary. This occurs via pollen tube. The
pollen tube grows out of the pollen grain, travels through the style, and
finally reaches the ovary. The fertilization In the flowering plant is shown in
the given figure.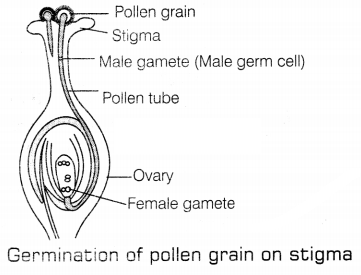
After fertilization, the zygote divides many times and forms
an embryo within the ovule. This ovule then develops a tough coat and gets
converted into a seed. The ovary rapidly grows and ripens as fruit. The seed
contains the future embryo that develops into a seedling under suitable
conditions. This process is known as germination. (3)
Or
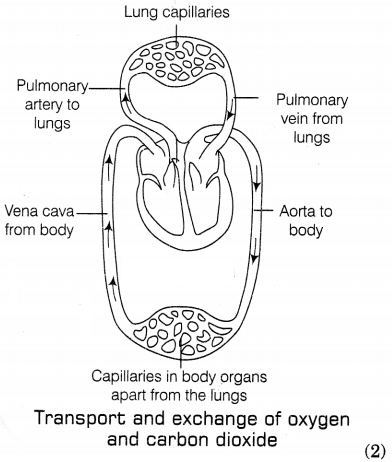
(a) During double
circulation in human beings, the blood passes twice through the heart during one
complete cycle of circulation. The double circulation includes the following
processes. (1)
- Pulmonary circulation: In this, the deoxygenated blood is pushed by the right ventricle to the lungs for oxygenation. The oxygenated blood then comes back to the left atrium of the heart through the pulmonary veins. (1)
- Systemic circulation: In this circulation, oxygenated blood from the left ventricle of the heart is passed to the different parts of the body through the aorta. Such a separation allows a highly efficient supply of oxygen to the body. This is useful in animals that have high energy needs such as birds and mammals including human beings, which constantly use energy to maintain their body temperature.
(b) During fermentation in yeast, ethanol is formed, while in
aerobic respiration CO2 and water are formed. The energy released in
the process of fermentation is also much less as compared to energy released in
aerobic respiration. (1)
Question 36.
(i) “A convex lens can form a magnified erect as well as
magnified inverted image of an object placed in front of it.” Draw a ray diagram
to justify this statement stating the position of the object concerning the lens
in each case.
(ii) An object of height 4 cm is placed at 20 cm from a concave
lens of focal length 10 cm. Use lens formula to determine the position of the
image formed.
OR
(i) To construct a ray diagram, we use two rays which are
so chosen that it is easy to know their directions after reflection from the
mirror. List two such rays and state the path of these rays after reflection in
case of concave mirrors. Use these two rays and draw a ray diagram to locate the
image of an object placed between the pole and the focus of a concave
mirror.
(ii) A concave mirror produces three times magnified image on a
screen. If the object is placed 20 cm in front of the mirror, how far is the
screen from the object?
Answer:
(i) Case I: Image formed is magnified and
erect when the object is placed between the optical centre and focus on a convex
lens.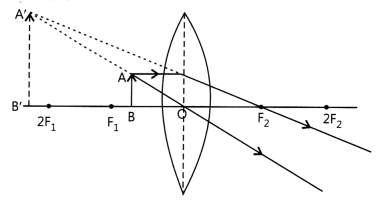
Case II: Image formed is magnified and inverted when the object is placed
between F and 2F of a convex lens.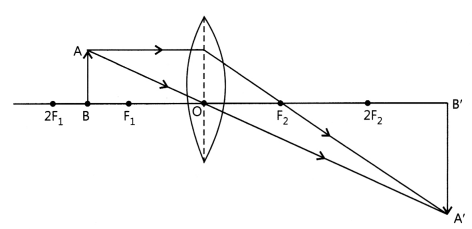
(ii) Given: u = – 20 cm, f = – 10 cm.
Using lens
formula,
\(\frac{1}{f}=\frac{1}{υ}-\frac{1}{u}\)
⇒
\(\frac{1}{υ}=\frac{1}{f}+\frac{1}{u}\)
⇒
\(\frac{1}{υ}=-\frac{1}{10}-\frac{1}{20}\)
⇒
\(\frac{1}{υ}=\frac{-2-1}{20}=\frac{-3}{20}\)
⇒ υ = \(\frac{-20}{3}\) cm
OR
(i) The following rays of light are usually used to locate the images
formed by a spherical mirror : The incident ray passing through the centre of
curvature: In this case, light after reflecting from the spherical mirror moves
back along the same path.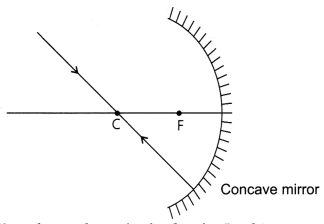
The ray incident obliquely to the principal axis: In this
case, the incident ray will be reflected back by the reflecting surface of the
spherical mirror obliquely, making equal angles with the principal axis.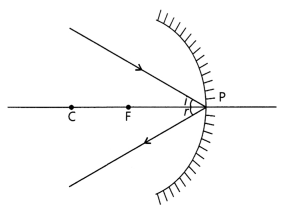
When the object is placed between the pole and the focus of
the concave mirror, an image is formed behind the mirror and is virtual, erect
and magnified.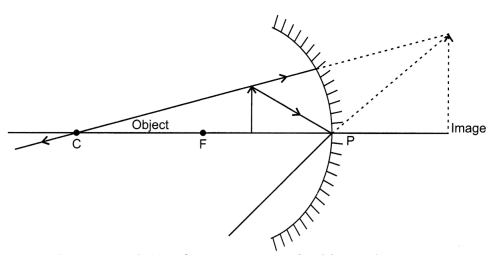
(ii) Given, Magnification, m = – 3 (As the image is real),
Object distance, u = – 20 cm
We have, m = –\(\frac{υ}{u}\)
⇒ – 3 = –
\(\left(\frac{υ}{-20}\right)\)
⇒ υ = – 60 cm
The image is located at 60 cm
in front of the mirror.
Thus, the screen is 40 cm from the object.
Section E
Questions No. 37 to 39 are case-based/data-based questions with 2 to 3 short sub-parts. Internal choice is provided in one of these sub-parts.
Question 37.
Abhishika observed that the cooking utensils of her kitchen
are becoming black and the flame of her burner becomes yellowish. She complained
about this to the gas company and they repaired it.
(a) What was the reason
for this type of flame?
(b) How can this problem affect our environment?
Or
What precautions should be taken to avoid this process?
Answer:
(a)
The inlets present in the burner get blocked due to which proper combustion does
not take place. This results in the yellow flame. (2)
(b) This process is
known as incomplete combustion and is very harmful to our environment. This
leads to the formation of oxides which are major pollutants. (2)
Or
To
prevent this situation, burners of gas or stores should be cleaned timely so
that inlets do not block. (2)
Question 38.
Stomata are tiny pores present on the surface of the leaves.
Massive amounts of gaseous exchange take place in the leaves through these pores
for photosynthesis. But it is important to note here that the exchange of gases
occurs across the surface of stems, roots, and leaves as well. Since large
amounts of water can also be lost through these stomata, the plant closes these
pores when it does not need carbon dioxide for photosynthesis. The opening and
closing of the pore is a function of the guard cells. The guard cells swell when
water flows into them, causing the stomatal pores to open. Similarly, the pore
closes if the guard cells shrink.
1. Take two healthy potted plants which are
nearly the same size.
2. Keep them in a dark room for three days.
3. Now,
place each plant on separate glass plates. Place a watch glass containing
potassium hydroxide by the side of one of the plants.
4. The potassium
hydroxide is used to absorb carbon dioxide.
5. Cover both plants with
separate bell jars.
6. Use Vaseline to seal the bottom of the jars to the
glass plates so that the set-up is air-tight.
7. Keep the plants in sunlight
for about two hours.
8. Pluck a leaf from each plant and check for the
presence of starch as in the above activity.
(a) What can be concluded from
the activity?
(b) What is the function of stomata?
(c) How do the guard
cells control the opening and closing of stomata?
OR
In the above
activity, do both the leaves show the same amount of starch?
Answer:
(a)
It shows that the amount of carbon dioxide affects the process and outcome of
photosynthesis.
(b) Massive amounts of gaseous exchange take place in the leaves through these pores for photosynthesis.
(c) The guard cells swell when water flows into them, causing the stomatal
pore to open. Similarly, the pore closes if the guard cells shrink.
OR
No,
both the leaves show the presence of a different amount of starch in the given
activity.
Question 39.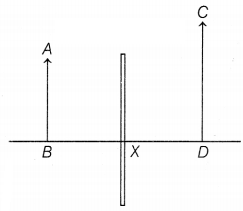
The above diagram shows that two students P and Q
experimented on finding the image formation by a mirror. They experimented with
different positions of objects and obtained different positions of images. AB,
CD, and X represent objects, images, and the optical device respectively.
(a)
Based on the text and data given in the above paragraph, identify the type of
optical device X.
(b) What will be the position of the image? If the object
is placed between the focus and center of curvature.
(c) If the object is
placed at a distance of 10 cm in front of the mirror X, then what will be the
distance of the image formed? [Take, magnification = -3]
Or
What will
happen, if the upper half of the concave mirror ‘X’ is covered?
Answer:
(a) In this case, the image formed is virtual, erect, and magnified. The image
is formed behind the optical device X. Hence, device X is a concave mirror. When
the object is placed between the pole and the focus of a concave mirror, the
image formed will be virtual, erect, magnified, and behind the mirror. (1)
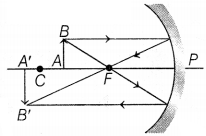
(b) Concave mirror X will form the magnified image of the object as the
object is placed between the focus and centre of curvature of the mirror.
(1)
(c) Given, object distance, u = -10 cm
Magnification, m =
-3
Now, m = \(-\frac{v}{u}\)
⇒ -3 = \(-\frac{v}{(-10)}\)
⇒ v = -30
cm
The negative sign shows that the image is real and in front of the mirror.
(2)
Or
If the upper half of the concave mirror is covered, the focal
length of the mirror and object distance do not change, then the brightness of
the image will be reduced. (1)
This is because the whole image will be formed
by the rays passing through the upper half portion but the intensity of the
image formed is proportional to the number of rays and hence the image will be
less brighter. (1)