CBSE Sample Papers for Class 10 Science Set-1
Class 10thCBSE Sample Papers for Class 10 Science Set-1
CBSE Sample Papers for Class 10 Science Set 1
Time: 3 Hours
Maximum Marks: 80
Instructions
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. A student is expected to attempt only one of these questions.
- Section A consists of 20 objective-type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 2 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer questions with 3 marks each. Answers to these questions should be in the range of 50 to 80 words.
- Section D consists of 3 Long Answer questions with 5 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 4 marks each with sub-parts.
Section A
Select and write the most appropriate option out of the four options given for each of the questions 1-20.
Question 1.
Pratyush took sulphur powder on a spatula and heated it. He
collected the gas evolved by inverting a test tube over it, as shown in the
figure below.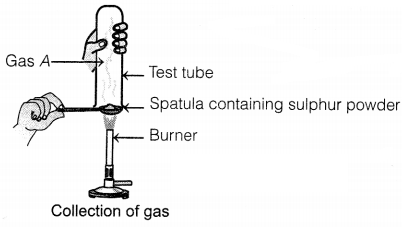
Identify gas A in the given experiment.
(a)
SO2
(b) SO3
(c) H2S
(d)
N2
Answer:
(a) SO2
The gas that evolved in the
given experiment is sulphur dioxide (SO2).
Question 2.
The colour of the solution observed after 30 minutes of
placing zinc metal to copper sulphate solution is
(A) Blue
(B)
Colourless
(C) Dirty green
(D) Reddish Brown
Answer:
(B)
Colourless
Detailed Answer:
Zinc is being more reactive than copper
displaces it from copper sulphate solution and form a colourless solution of
zinc sulphate.
Question 3.
A molecule of ammonia (NH3) has
(a) only single
bonds
(b) only double bonds
(c) only triple bonds
(d) two double bonds
and one single bond
Answer:
(a) only single bonds
A molecule of ammonia
(NH3) has only single bonds and these are covalent.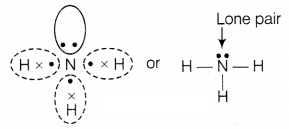
Question 4.
On adding dilute sulphuric acid to a test tube containing a
metal ‘X’, a colourless gas is produced when a burning match stick is brought
near it. Which of the following correctly represents metal ‘X’?
(A)
Sodium
(B) Zinc
(C) Copper
(D) Silver
Answer:
(A) Sodium
Detailed Answer: The metal X is sodium. On reaction with sodium metal to the
sulphuric acid, sodium replaces the hydrogen ion and forms sodium sulphate and
hydrogen gas. When a lighted matchstick is brought near the mouth of the test
tube containing hydrogen gas, it burns with a pop-up sound.
H2SO4 + 2Na → Na2SO4 +
H2
Question 5.
Gold is used for making jewellery. What are the properties of
gold that make it a suitable metal for making jewellery?
(a) Ductility
(b)
Malleability
(c) Lustrous
(d) All of these
Answer:
(d) All of
these
Gold has all given properties that make it suitable for making
jewellery. Gold is ductile, malleable, and lustrous. It can be drawn into thin
sheets and wires and has a shiny appearance.
Question 6.
An element with atomic number will form a basic oxide.
(A)7
(2,5)
(B) 17 (28,7)
(C) 142,84)
(D) 11 (28,1)
Answer:
(D) 11
(28,1)
Detailed Answer:
Metals form basic oxide and can have 1 to 3
electrons in their outermost shell while non-metals can form acidic oxides and
have 4 to 8 electrons in their outermost shell. Hence, element with atomic
number 11 (electronic configuration 2, 8,1) is metal (Na) and it will form basic
oxide.
Question 7.
Which among the following are unsaturated
hydrocarbons?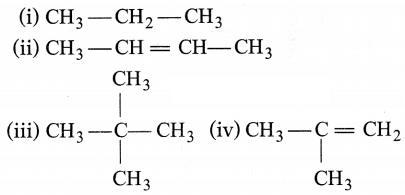
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and
(iv)
(d) (ii), (iii) and (iv)
Answer:
(c) Unsaturated hydrocarbons have
double or triple bonds in their structure. Both (ii) and (iv) have double
carbon-carbon bonds in their structures.
Question 8.
Generally, food is broken and absorbed within the body of
organisms. In which of the following organisms is it done outside the body?
(A) Amoeba
(B) Mushroom
(C) Paramoecium
(D) Lice
Answer:
(B)
Mushroom
Detailed Answer:
Mushroom is a fungus and fungi are an organism
that breaks down food into simpler chemicals outside the body before absorbing
it.
Question 9.
A plant is kept in the dark for two days. A leaf from this
plant is used in an experiment to investigate the effect of two factors on
photosynthesis as shown in the diagram.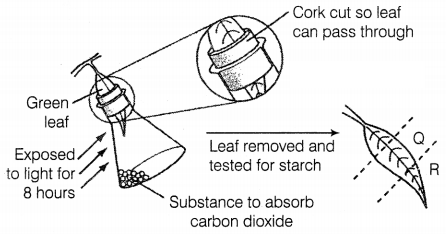
What are the colours of Q and R, when the leaf is tested for
starch, using an iodine solution?
| Q | R |
| (a) Blue/Black | Brown |
| (b) Brown | Brown |
| (c) Blue/Black | Blue/Black |
| (d) Brown | Blue/Black |
Answer:
(b) Q – Brown, R – Brown
The colours of both Q and R regions
when tested with iodine solution appeared brown. This indicates that there was
no starch in both regions. In both regions Q and R photosynthesis did not occur,
since part Q of the leaf was covered by the cut part of the cork, while part R
was exposed to KOH kept in the flask to absorb CO2. In the absence of
light (for Q) and CO2 (for R), photosynthesis did not occur, thus
starch was not formed and regions Q and R tested negative with iodine (A
positive test for starch converts the leaf blue-black with iodine). (1)
Question 10.
A farmer wants to grow banana plants genetically similar
enough to the plants already available in his field. Which one of the following
methods would you suggest for this purpose?
(A) Regeneration
(B)
Budding
(C) Vegetative propagation
(D) Sexual reproduction
Answer:
(C) Vegetative propagation
Detailed Answer:
Farmers can cultivate banana
crop by vegetative propagation. It is asexual mode of reproduction. It is done
by propagating the rhizome of banana. The rhizome has the ability to give rise
to new banana plant.
Question 11.
The correct sequence of organs in the male reproductive
system for the transport of sperm is
(a) testis → vas deferens → urethra
(b) testis → ureter → urethra
(c) testis → urethra → ureter
(d) testis →
vas deferens → ureter
Answer:
(a) testis → vas deferens → urethra
Sperms formed in testis are delivered through the vas deferens which join with
another tube called the urethra coming from the urinary bladder.
Question 12.
A sportsman, after a long break of his routine exercise,
suffered muscular cramps during a heavy exercise session. This happened due
to:
(A) lack of carbon dioxide and formation of pyruvate.
(B) presence of
oxygen and formation of ethanol.
(C) lack of oxygen and formation of lactic
acid.
(D) lack of oxygen and formation of carbon dioxide.
Answer:
(C)
lack of oxygen and formation of lactic acid.
Detailed Answer:
During heavy
exercise, the energy demand is high but the supply of oxygen to produce energy
is limited. Therefore, anaerobic respiration takes places in the muscles cells
to fulfill the energy demand. This anaerobic breakdown of glucose leads to the
formation of lactic add in musdes. The accumulation of lactic add in musdes
leads to muscle cramps.
Question 13.
An object is placed in front of a convex mirror. Its image is
formed
(a) at a distance equal to the object distance in front of the
mirror.
(b) at twice the distance of the object from the mirror.
(c) half
the distance of the object in front of the mirror.
(d) behind the mirror and
its position varies according to the object’s distance.
Answer:
(d) behind
the mirror and its position varies according to the object’s distance.
An
object is placed in front of a convex mirror, its image is formed behind the
mirror and its position varies according to the object’s distance.
Question 14.
When light enters the atmosphere it strikes on extremely fine
particles, which deflect the rays of light in all possible directions, This is
due to-
(A) reflection of light
(B) atmospheric refraction
(C)
scattering of light
(D) dispersion of light
Answer:
(C) scattering of
light
Detailed Answer:
When light enters the atmosphere, it strikes on
extremely fine particles. This deflects the rays of light in all possible
directions and the phenomenon is termed as scattering of light.
Question 15.
Which statement about the genotypes of 1 organism is
correct?
(a) Dominant alleles are only found in homozygous
(b) One
recessive allele always causes a recessive phenotype
(c) Recessive phenotypes
must be homozygous
(d) The dominant phenotype must be heterozygous
Answer:
(c) Recessive phenotypes must be homozygous
A recessive trait
requires a homozygous condition to express itself.
Question 16.
Which of the following features relates to biodegradable
substances?
(A) Broken down by biological processes
(B) Remain inert
(C) Persist in the environment for a long time.
(D) May harm the
ecosystem.
Answer:
(A) Broken down by biological processes
Detailed
Answer:
Biodegradable materials are substances that can be decomposed or
broken down by microorganisms or decomposers.
Direction (Nos. 17-20) consists of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A).
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true.
Question 17.
Assertion (A): Graphite is slippery to the touch.
Reason
(R): The various layers of carbon atoms in graphite are held together by weak
Vander Waals’ forces.
Answer:
(a) Both A and R are true and R is the
correct explanation of A.
A graphite crystal consists of various layers of
carbon atoms in which each carbon atom is joined to three other atoms by strong
covalent bonds. The various layers of carbon atoms in graphite are held together
by weak van der Waals forces making it slippery to touch.
Question 18.
Assertion: The probability of survival of an organism
produced through sexual reproduction is more than that of an organism produced
through asexual mode.
Reason: Variations provide advantages to individuals
for survival.
Answer:
(A) Both A and R are true and R is the correct
explanation of A.
Detailed Answer:
The probability of survival of an
organism produced through sexual reproduction is more than that of an organism
produced through asexual mode as the combination of genes allows for different
characteristics. Variations provide advantages to individuals for survival.
Question 19.
Assertion (A): Blood pressure is much greater in arteries
than veins.
Reason (R): The force exerted by blood against the wall of the
vessel is called blood pressure.
Answer:
(b) Both A and R are true, but R
is not the correct explanation of A.
Blood pressure is much greater in
arteries because of the faster pumping action of the heart which sends blood
rapidly in arteries. (1)
Question 20.
Assertion: Biodegradable substances result in the formation
of compost and natural replenishment.
Reason: It is due to the breakdown of
complex inorganic substances into simple organic substances.
Answer:
(C) A
is true but R is false.
Detailed Answer:
Recycling of organic wastes like
vegetable peels, waste food, leaves, etc., by burying them in compost pits is
called composting. Hence, biodegradable substances result in the formation of
compost and natural replenishment by the action of small organisms like baderia
and fungi. Decomposition is the physical and chemical breakdown of complex
organic matter into simple inorganic substances.
Section B
Questions No. 21 to 26 are Very Short Answer Questions.
Question 21.
A solid limestone is burnt to form quicklime and a gas.
Define the reaction involved and also write its chemical equation.
Answer:
A reaction in which a single reactant breaks down to form two or more
products is known as a decomposition reaction. The chemical equation can be
represented as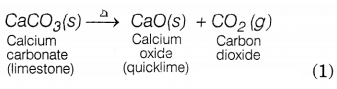
Question 22.
State the post-fertilization changes that lead to fruit
formation in plants.
Answer:
After fertilization, the zygote divides
several times to form an embryo within the ovule.
- The ovule develops a tough coat and is gradually converted into a seed.
- The ovary grows rapidly and ripens to form a fruit.
- The petals, sepals, stamens, style and stigma may shrivel and fall off.
Question 23.
An acid is secreted in our stomach. Why?
Or
Differentiate between respiration in plants and respiration in animals.
Answer:
HCl (Hydrochloric Acid) is the acid secreted inside the stomach. It
is important for the body because
(i) It makes the medium inside the stomach
acidic, which is necessary for the activation of an enzyme called pepsin.
(1)
(ii) It also kills any bacteria, entering the stomach along with food.
(1)
Or
| Respiration in Plants | Respiration in Animals |
| (i) Plants do not possess a respiratory system. | (i) Animals have respiratory systems. |
| (ii) Diffusion of respiratory gases directly takes place into the cells. | (ii) The respiratory gases are transported upto the tissue cells. |
Question 24.
The refractive indices of three media are given:
| Medium | Refractive Index |
| A | 1.6 |
| B | 1.8 |
| C | 1.5 |
Aray of light is travelling from A to B and another ray is travelling from B
to C.
(a) In which of the two cases the refracted ray bends towards the
normal?
(b) In which case does the speed of light increase in the second
medium? Give reasons for your answer.
Answer:
(a) When light travels from
an optically rarer medium to an optically denser medium, it moves towards the
normal. Since n8 > na hence the light ray will bend
towards the normal on passing from medium A to B.
(b) The speed of the light
will increase when the light travels from B to C, Since nc <
n8 and v = (c/n), the speed of light ray will increase in the second
medium.
Question 25.
The given figure shows three resistors.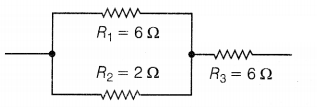
Find the combined resistance.
Or
For the current
carrying solenoid as shown below, draw magnetic field lines and give a reason to
explain that out of the three points A, B, and C at which point the field
strength is maximum and at which point it is minimum.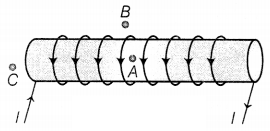
Answer:
Here, R1 is parallel to
R2
For parallel combinations,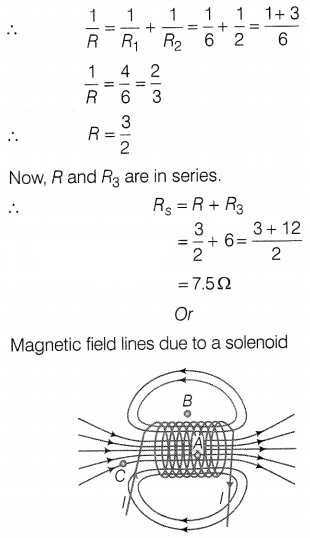
In the case of an ideal solenoid, the magnetic field strength
is maximum at point A and is minimum or zero at point B. This is because the
magnetic field is strong, where magnetic field lines are crowded, and is weak,
where magnetic field lines are far apart. At point C, the density of the field
lines is less than that of point A but greater than that of point B. So, the
order of the magnetic field at points A, B, and C is BB <
BC < BA. (2)
Question 26.
Study the food chain given below and answer the questions
that follow: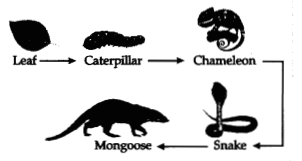
(a) If the amount of energy available at the third trophic
level is 100 joules, then how much energy will be available at the producer
level? Justify your answer.
(b) Is it possible to have 2 more trophic levels
in this food chain just before the fourth trophic level? Justify your
answer.
Answer:
(a) 10000J because only 10 % of energy is available for
the next trophic level.
(b) No, since the loss of energy at each step is so
great that very little usable energy will remain after 4 trophic levels.
Section C
Questions No. 27 to 33 are Short Answer Questions.
Question 27.
State which of the following chemical reactions will take
place or which will not, giving the suitable reason for each.
(a) Zn (s) +
CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
(b) Fe (s) +
ZnSO4 (aq) → FeSO4 (aq) + Zn (s)
(c) Zn (s) +
FeSO4 (aq) → ZnSO4 (aq) + Fe (s)
Answer:
(a) Zinc
displaces copper from copper sulphate because zinc is more reactive than copper
as it is placed above copper in the reactivity series of metals. (1)
(b) This
reaction will not occur because iron is less reactive than zinc as it is placed
below zinc in the reactivity series of metals. (1)
(c) Zinc displaces iron
because zinc is more reactive than iron as it is placed above iron in the
reactivity series of metals. (1)
Question 28.
An element ‘M’ with electronic configuration 2, 8, 3 combines
separately with Cl–, SO-2 anions. Write the chemical
formulae of the compounds formed. Predict with the suitable reason the nature of
the bond formed by element ‘M’ general. How will the electrical conductivity of
the compounds formed vary concerning ‘M’?
Answer:
MCl3 ;
M2(SO4)3
M in general forms ionic bond. It
can acquire a stable electronic configuration of neon (2, 8) by losing its three
valence electrons to form M3+, cation.
Compounds formed will
conduct electricity in liquid / molten state but not in solid state in contrast
to ‘M’.
OR
A reddish-brown metal ‘X’, when heated in air, gives a black compound ‘Y’,
which when heated in presence of H2, gas gives ‘X’ back. ‘X’ is
refined by the process of electrolysis; this refined form of ‘xX’ is used in
electrical wiring. Identify ‘x’ and ’Y’. Draw a well-labeled diagram to
represent the process of refining ‘X’.
Answer:
(a) ‘X’ – Copper/ Cu and
‘Y’ – CuO
(b) Diagram to represent the process of refining of ‘X’.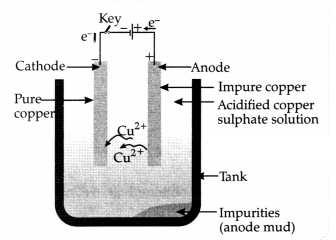
Question 29.
A green stemed rose plant denoted by GG and a brown stemed
rose plant denoted by gg are allowed to undergo a cross with each other. List
your observations regarding
(a) the colour of the stem in their F1
progeny
(b) the percentage of brown stemmed plants in F2 progeny
if F1 plants are self-pollinated.
(c) the ratio of GG and Gg in
the F2 progeny.![]()
Answer:
(a)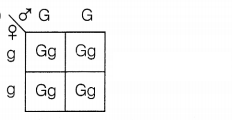
All the plants will be with green stems in F1
progeny.
(b)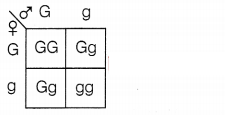
25% of plants will be brown-stemmed. (1)
(c) 25% GG
50%
Gg
Question 30.
What is the probability of a girl or a boy being born in a
family? Justify your answer.
Answer:
There are 50% chances that a girl may
be born and 50% chances that a boy may be bom.
It can be explained as
follows:
(i) Most human chromosomes have a maternal copy and a paternal copy.
We have 22 such chromosomes. One pair of chromosomes called sex chromosomes is
odd in not always being a perfect pair. Women have a perfect pair of sex
chromosomes, both called X. (XX)
(ii)But men have a mismatched pair of sex
chromosomes in which one is normal sized – X chromosome while the other is a
short one called Y chromosome. (XY)
(iii) A child receives one chromosome
from mother which is essentially X chromosome.
(iv) A child who inherits an X
chromosome from her father will be a girl, and one who inherits a Y chromosome
from him will be a boy.
Question 31.
Rohit wants to have an inverted image of an object using a
concave mirror. If he kept the object at a distance of 50 cm from the mirror and
the image is formed at a distance of 30 cm from the mirror, then
(a) specify
the range of focal length of the concave mirror.
(b) draw the ray diagram to
show image formation in this case.
(c) state the magnification produced by a
concave mirror.
Answer:
(a) Given, object distance, u = -50 cm, image
distance, v = -30 cm
From the mirror formula,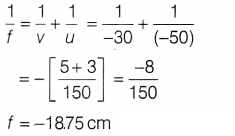
(b) The ray diagram for image formation by concave mirror is
shown below.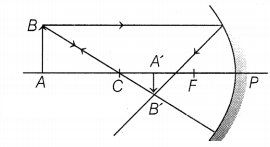
(c) Magnification of mirror,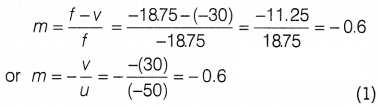
Question 32.
(a) State the law that explains the heating effect of current
concerning the measurable properties in an electrical circuit.
(b) List the
factors on which the resistance of a conductor depends.
Answer:
(i)
Joule’s law of heating states that the heat dissipated across a resistor is
directly proportional to:
(a) The square of the current flowing through
it.
(b) The resistance of the conductor.
(c) Duration of flow of
current.
H= l2Rt (alternative answer).
(ii) Resistance of a
conductor depends on:
(a) the length of the conductor
(b) the area of the
cross section
(c) nature of material
(d) temperature of the conductor
Question 33.
A current is allowed to flow through a solenoid. State your
observations for the following cases and give reasons for the same in each
case.
(a) The solenoid behaves like a magnet.
(b) The solenoid is a
temporary magnet.
Answer:
(a) A solenoid is a long coil of circular loops
of insulated copper wire. Magnetic field lines are produced around the solenoid
when a current is allowed to flow through it. The field lines produced by it are
similar to the field lines produced by a bar magnet which can be seen in the
figure.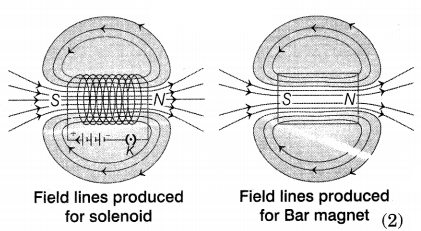
(b) The solenoid behaves like a temporary magnet which is
formed by placing a soft iron core inside it. The magnetism of this magnet lasts
so long as there is current flowing through it and loses its magnetic property
when the current in the solenoid is stopped. It is also known as an
electromagnet. (1)
Section D
Questions No. 34 to 36 are Long Answer Questions.
Question 34.
(a) Rehmat classified the reaction between Methane and
Chlorine in presence of sunlight as a substitution reaction. Support Rehmat’s
view with suitable justification and illustrate the reaction with the help of a
balanced chemical equation.
(b) Chlorine gas was prepared using electrolysis
of brine solution. Write the chemical equation to represent the change. Identify
the other products formed in the process and give one application of each.
Answer:
(a) Rehmat’s observation is correct as the hydrogen atoms are
substituted by hetero atom, i.e., Cl.
CH4 + Cl2 →
CH3Cl + HCl (in the presence of sunlight)
(Any other relevant
equation in the chain reaction)
(b) 2NaCl(aq) + 2H2O(l) →
2NaOH(aq) + Cl2(g) + H2(g)
(OR)
\(\left[\begin{array}{l}
\mathrm{NaCl} \rightarrow
\mathrm{Na}^{+}+\mathrm{Cl}^{-} \\
2 \mathrm{Cl}^{-} \rightarrow
\mathrm{Cl}_2+2 \mathrm{e}^{-} \text {(At anode) } \\
\mathrm{H}_2 \mathrm{O}
\rightarrow \mathrm{H}^{+}+\mathrm{OH}^{-} \\
2 \mathrm{H}^{+}+2
\mathrm{e}^{-} \rightarrow \mathrm{H}_2 \text { (At cathode) } \\
\mathrm{Na}^{+}+\mathrm{OH}^{-} \rightarrow \mathrm{NaOH}
\end{array}\right]\)
Other products and usages
Sodium hydroxide/ NaOH/
Caustic soda
Hydrogen Uses: (any one each)
Sodium hydroxide/ NaOH/ Caustic
soda:
- Degreasing of metals
- Preparation of soaps and detergents
- Paper making
- Artificial fibres
Hydrogen:
- Fuels
- Margarine
- Manufacture of ammonia for fertilisers
OR
Raina while doing certain reactions observed that heating of substance ‘X’ with vinegar like smell with a substance ‘Y’ (which is used as an industrial solvent) in presence of conc. Sulphuric acid on a water bath gives a sweet- smelling liquid ‘Z’ having molecular formula C2H2O2. When heated with caustic soda (NaOH), ‘2’ gives back the sodium salt of and the compound ‘Y’.
Identify ‘x’, ‘Y’, and ‘Z’. Illustrate the changes with the help of suitable
chemical equations.
Answer: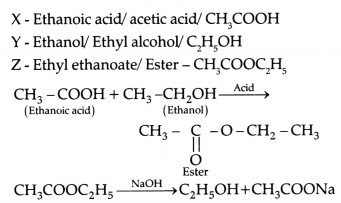
Question 35.
Given below are certain situations. Analyse and describe its
possible impact on a person.
(a) The fallopian tube of a female is
plugged.
(b) Ovaries are not functional.
(c) The urethra is not
functioning properly.
(d) Testosterone is not produced by the testis.
(e)
Oestrogen and progesterone are not secreted by the ovaries.
Or
(a) What
are animal hormones? List their two characteristics.
(b) Name the
hormone,
(i) which brings change in male humans during the beginning of
adolescence.
(ii) which coordinates the level of sugar in blood?
Answer:
(a) If the fallopian tube of a female is plugged then the egg will
not be able to reach the uterus. Therefore, fertilization will not take
place.
(b) If ovaries are not functional in the females then it will not
produce ova or egg cells, which are necessary for the process of
fertilization.
(c) As urethra is a common passage for both sperm and urine.
So, if the urethra is not functioning properly, it will affect the transport of
sperm.
(d) The testosterone also known as male sex hormone is produced by the
testis. So if the testis does not produce testosterone, then it will not bring
sexual maturation at puberty.
(e) Oestrogen and progesterone are the female
sex hormones. These bring about sexual character during puberty and also play a
major role in menstruation. (1 × 5)
Or
(a) Hormones are chemical
substances, which control and coordinate the activities performed by
organisms.
Characteristics of Hormones:
(i) They are poured directly into
the bloodstream and are carried throughout the body by the circulatory
system.
(ii) They act only on the specific target organs.
(b) (i)
Testosterone produced by the testes regulates the changes in males during the
adolescence period.
(ii) Insulin (decreases blood sugar) and glucagon
(increases blood sugar), secreted by the pancreas coordinate the sugar level in
blood.
Question 36.
The given image shows a thin lens of focal length 5m.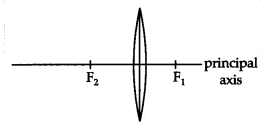
(a) What is the kind of lens shown in the above figure?
(b) Ifareal inverted image is to be formed by this lens at a distance of 7m from
the optical centre, then show with calculation where should the object be
placed?
(c) Draw a neatly labelled diagram of the image formation mentioned
in (ii)
Answer:
(i) Convex lens
(ii) 1/f = 1/v – 1/u
In this case, v
= 7m and f = 5m.
Putting the values in the equation we get
\(\begin{gathered}
\frac{1}{5}=\frac{1}{7}-\frac{1}{u} \\
\frac{1}{u}=\frac{1}{7}-\frac{1}{5}=\frac{5-7}{35}=\frac{-2}{35} \\
u=-\frac{35}{2}=-17.5 \mathrm{~m}
\end{gathered}\)
The object will be
placed 17.5 m on the left of the convex lens.
(iii)
(two rays, arrows, object placed beyond 2f on the left)
OR
A 10cm long pencil is placed 5 cm in front of a concave mirror having a
radius of curvature of 40 cm.
(a) Determine the position of the image formed
by this mirror.
(b) What is the size of the image?
(c) Draw a ray diagram
to show the formation of the image as mentioned in the part (i).
Answer: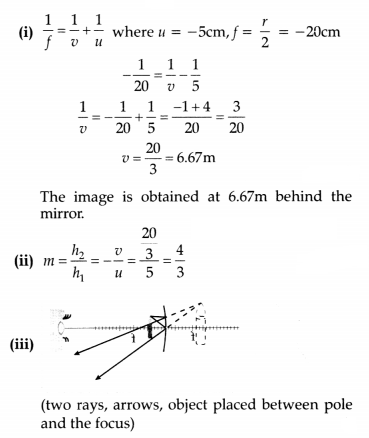
Section E
Questions No. 37 to 39 are case-based/data-based questions with 2 to 3 short sub-parts. Internal choice is provided in one of these sub-parts.
Question 37.
The table given below shows the hints given by the quiz
master in a quiz.
| Hints |
| (i) ‘X’ metal acts as a good reducing agent. |
| (ii) ‘X’ reduces ‘Y’ and MnO2. |
| (iii) The reaction with ‘Y’ is used for joining broken railway tracks. |
Based on the above hints answer the following questions.
(a) Identify ‘X’
and ‘Y’.
(b) Write all the chemical reactions.
Or
Name the reaction
which occurs in step 3. Define it.
Answer:
(a) X = Aluminium (Al), Y =
Iron (III) oxide (Fe2O3) (2)
(b) 3MnO2 (s) +
4Al (s) → 3Mn (l) + 2Al2O3 (s) + Heat
Fe2O3 (s) + 2Al (s) → 2Fe (l) +
Al2O3 (s) + Heat (2)
Or
The reaction involved is a
thermite reaction. The reaction of metal oxide to form metal by using aluminium
powder as a reducing agent is known as a thermite reaction. (2)
Question 38.
Figures (a) to (d) given below represent the type of ear
lobes present in a family consisting of 2 children – Rahul, Nisha and their
parents.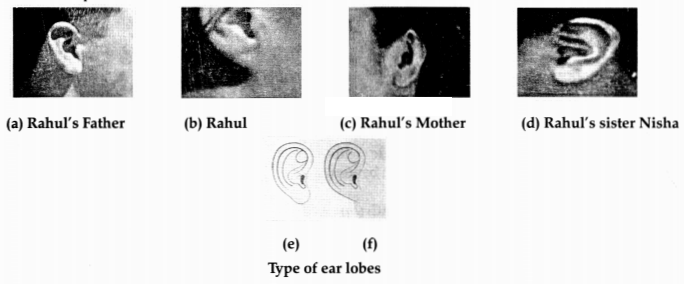
Excited by his observation of different types of ear lobes
present in – his family, Rahul surveyed the type of ear lobes found {Figure (e)
and (f)} in his classmates. He found two types of ear lobes in his classmates as
per the frequency given below:
| Sex | Free | Attached |
| Male | 36 | 14 |
| Female | 31 | 19 |
Based on the above data answer the following questions.
(a) Which of the
two characteristics – ‘free ear lobe’ or ‘attached ear lobe’ appears to be
dominant in this case? Why?
(b) Is the inheritance of the free ear lobe
linked with sex of the individual? Give reason for your answer.
(c) What type
of ear lobe is present in father, mother, Rahul and his sister Nisha? Write the
genetic constitution of each of these family members which explains the
inheritance of this character in this family?
(Gene for Free ear lobe is
represented by F and gene for attached ear lobe is represented by f for writing
the genetic constitution).
Answer:
(a) Free ear lobe is dominant because
it is found in a large majority of the population.
(b) No. It is not
sex-linked. As per the data of the family as well as the class, it is indicated
that free ear lobe is present in males as well as in females
(c) Father – Ff
(free ear lobe), Mother – Ff (free ear lobe), Rahul – ff (attached ear lobe) and
Nisha – Ff (free ear lobe).
OR
Suresh’s parents have attached ear lobes. What type of ear lobe can be seen
in Suresh and his sister Siya? Explain by giving the genetic composition of
all.
Answer:
Suresh’s father- ff (attached ear lobe), mother – ff
(attached ear lobe), Suresh – ff (attached ear lobe), Siya – ff (attached ear
lobe). If both parents have recessive character, then all the children can have
recessive character only.
Question 39.
Vinita and Ahmed demonstrated a circuit that operates the two
headlights and the two sidelights of a car, in their school el exhibition. Based
on their demonstrated circuit, answer the following questions.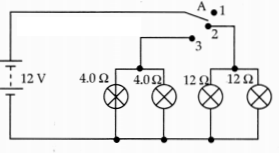
(i) State what happens when switch A is connected to
(a)
Position 2
(b) Position 3
(ii) Find the potential difference across each
lamp when lit.
(iii) Calculate the current
(a) in each Ω lamp when
lit.
(b) In each Ω lamp when lit.
Answer:
(i) (a) 12 Ω lamps (only)
ON.
(b) 4 Ω lamps (only) ON
(ii) 12 V for both sets of lamps because all
of them are in parallel.
(iii) (a) 12 Ω lamps are ON when the wire is
connected to position 2.
voltage across both 12 Ω lamps = 12 V
V = IR
(Ohm’s law)
I = \(\frac{V}{R}=\frac{12}{12}\) = 1A.
(b) 4 Ω lamps are ON
when the wire is connected to position 3.
Voltage across both 4 Ω lamps = 12
V
V = IR (Ohm’s law).
I = \(\frac{V}{R}=\frac{12}{4}\) = 3A.
OR
(iv) Show, with calculations, which type of lamp, 4.0 Ω or 12.Ω, has the
higher power.
Answer:
P = V2/R
All lamps are in parallel and
hence same V for all lamps.
For 4 Ω lamps → P = \(\frac{12 \times 12}{4}\) =
36W
For 12 Ω lamps → P = \(\frac{12 \times 12}{12}\) = 12W
Hence 4 Ω lamps
will have higher power.
Question 39.
Vinita and Roshan demonstrated a circuit that operates the
two headlights and the two sidelights of a car, in their school exhibition.
Based on their demonstrated circuit, answer the following questions.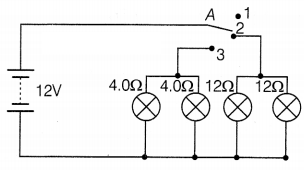
(a) State what happens when switch A is connected to
(i)
Position 2
(ii) Position 3
(b) Find the potential difference across each
lamp when lit.
(c) Calculate the current
(i) in each 12 Ω lamp when
lit.
(ii) in each 4 Ω lamp when lit.
Or
Show, with calculations, which
type of lamp, 4.0 Ω or 12 Ω, has the higher power.
Answer:
(a) (i) 12 Ω
lamp (only) on
(ii) 4 Ω lamps (only) on (1)
(b) 12V for both sets of lamps and all of them are in parallel. (1)
(c) (i) 12 Ω lamps are on when the wire is connected to position 2.
The
voltage across both 12 Ω lamps = 12 V
V = IR
I = \(\frac{V}{R}\)
=
\(\frac{12}{12}\)
= 1A
(ii) lamps are on when the wire is connected to
position 3.
The voltage across both 4 Ω lamps = 12 V
V = IR
I =
\(\frac{V}{R}\)
= \(\frac{12}{4}\)
= 3A (2)
Or
Power, P =
V2/R
All lamps are in parallel and hence same V for all lamps.
For 4 Ω lamps, P = \(\frac{12 \times 12}{4}\) = 36W
For 12 Ω lamps, P =
\(\frac{12 \times 12}{12}\) = 12W
Hence, 4 Ω lamps will have higher power.
(2)