Periodic Classification of Elements
Class 10th Science Chapter MCQs
Periodic Classification of Elements Class 10 MCQs Questions
Question 1.
Which one of the following does not increase while moving down
the group of the periodic table?
(a) Atomic radius
(b) Metallic
character
(c) Valence
(d) Number of shells in an
element.
Answer
Answer: (c) Valence
Question 2.
On moving from left to right in a period in the periodic
table, the size of the atom
(a) increases
(b) decreases
(c) does not
change appreciably
(d) first decreases and then
increases.
Answer
Answer: (b) decreases
1. Newlands relation is called
(a) Musical Law
(b) Law of Octaves
(c) Periodic Law
(d) Atomic Mass Law
Answer
Answer: b
2. Upto which element, the Law of Octaves was found applicable?
(a)
Oxygen
(b) Calcium
(c) Cobalt
(d)
Potassium
Answer
Answer: b
3. In Mendeleev’s Periodic Table, gaps were left for the elements to be
discovered later. Which of the following elements found a place in the Periodic
Table later?
(a) Chlorine
(b) Silicon
(c) Oxygen
(d) Germanium
Answer
Answer: d
4. At the time of Mendeleev, the number of elements known was
(a) 63
(b) 65
(c) 62
(d) 64
Answer
Answer: a
5. The properties of eka-aluminium predicted by Mendeleev are the same as the
properties of later discovered element:
(a) Scandium
(b) Germanium
(c)
Gallium
(d) Aluminium
Answer
Answer: c
6. An atom of an element has the electronic confi-guration 2,8,2. To which
group does it belong?
(a) 4th group
(b) 6th
group
(c) 3rd group
(d) 2nd
group
Answer
Answer: d
7. The arrangement of elements in the Modem Periodic Table is based on
their
(a) increasing atomic mass in the period
(b) increasing atomic
number in the horizontal rows
(c) increasing atomic number in the vertical
columns
(d) increasing atomic mass in the
group
Answer
Answer: b
8. Where would you locate the element with electronic configuration 2, 8 in
the Modern Periodic Table?
(a) Group 8
(b) Group 2
(c) Group 18
(d)
Group 10
Answer
Answer: c
9. Element ‘X’ forms a chloride with the formula XCl2, which is a
solid with high melting point. X would most likely be in the same group of the
periodic table as:
(a) Si
(b) Mg
(c) Al
(d)
Na
Answer
Answer: b
10. Which of these belong to the same period?
| Element | A | B | C |
| Atomic number | 2 | 10 | 5 |
(a) A, B
(b) B, C
(c) C, A
(d) A, B and
C
Answer/ Explanation
Answer: b
Explaination: Reason. B= 10 (2, 8), C = 5 (2, 3) Both have 2
periods.
11. Carbon belongs to the second period and Group 14. Silicon belongs to the
third period and Group 14. If atomic number of carbon is 6, the atomic number of
silicon is
(a) 7
(b) 14
(c) 24
(d)
16
Answer
Answer: b
12. Pick out the chemically most reactive elements from the given triads.
Li, Na, K F, Cl, Br
(a) Li and F
(b) Li and Br
(c) K and F
(d) K and
Br
Answer
Answer: c
13. What is the atomic number of element of period 3 and group 17 of the
Periodic Table?
(a) 10
(b) 4
(c) 17
(d)
21
Answer
Answer: c
14. Which one of the following statements is not correct about the trends in
the properties of the elements of a period on going from left to right?
(a)
The oxides become more acidic
(b) The elements become less metallic
(c)
There is an increase in the number of valence electrons
(d) The atoms lose
their electrons more easily
Answer
Answer: d
15. The elements A, B and C belong to groups 1, 14 and 17 respectively of the
Periodic Table. Which two elements will form ionic compounds?
(a) A and B
(b) A and C
(c) B and C
(d) None
Answer
Answer: b
16. An element X from group 2 of the Periodic Table reacts with Y from group
17 to form a compound. Give the formula of the compound.
(a)
XY2
(b) XY
(c) X2Y
(d)
(XY)2
Answer
Answer: a
17. A metal ‘M’ is in the first group of the Periodic Table. What will be the
formula of its oxide?
(a) MO
(b) M2O
(C) M2O3
(d) MO2
Answer
Answer: b
18. Name the neutral atom in the Periodic Table which has the same number of
electrons as K+ and Cl-.
(a) Helium
(b) Argon
(c) Neon
(d)
Krypton
Answer
Answer: b
19. An element X combines with oxygen to form an oxide XO. This oxide is
electrically con¬ducting. Write the formula of the compound formed when X reacts
with chlorine.
(a) XCl3
(b) XCl
(c) XCl2
(d)
XCl5
Answer
Answer: c
20. An element X has mass number 40 and contains 21 neutrons in its atom. To
which group of the Periodic Table does it belong?
(a) Group 1
(b) Group
4
(c) Group 2
(d) Group 3
Answer/ Explanation
Answer: a
Explaination: Reason. e = 19 (2, 8, 8,1)
21. Consider the following elements
20Ca, 8Or
18Ar, 16S, 4Be, 2He
Which of the
above elements would you expect to be in group 16 of the Periodic Table?
(a)
20Ca and 16S
(b) 20Ca and 8O
(c) 18Ar and 16S
(d) 8O and
16S
Answer
Answer: d
22. An element ‘A’ belongs to the third period and group 16 of the Periodic
Table. Find out the valency of A.
(a) Valency = 6
(b) Valency = 2
(c)
Valency = 1
(d) Valency = 3
Answer
Answer: b
23. Which one of the following statements is not correct about the trends in
the properties of the elements of a group on going down in a group?
(a) The
chemical reactivity of metals increases.
(b) The metallic character of
elements increases.
(c) The size of the atom increases.
(d) The valence
electrons increase.
Answer
Answer: d
24. Which of the following set of elements is written in order of their
increasing metallic character?
(a) Na Li K
(b) C Q N
(c) Mg Al Si
(d) Be Mg Ca
Answer
Answer: d
25. The atom of an element has electronic con-figuration 2, 8, 7. To which of
the following elements would it be chemically similar?
(a) N(7)
(b)
P(15)
(c) Na(11)
(d) F (9)
Answer
Answer: d
Explaination: Reason. Both have same number of valence
electrons
26.
An atom of an element has the electronic configuration 2, 8,
2. To which group does it belong?
(a) 4th group
(b) 6th group
(c) 3rd
group
(d) 2nd group
Answer
Answer: (d) 2nd group
27.
In modern periodic table, elements are arranged according to
their
(a) atomic weight
(b) density
(c) atomic number
(d) melting
point
Answer
Answer: (c) atomic number
28.
In which group are inert elements placed? ‘
(a) Group 8
(b) Group 10
(c) Group 1
(d) Group 18
Answer
Answer: (d) Group 18
29.
Which of the following sets of atomic number belong to that of
alkali metals?
(a) 1, 12, 30, 4, 62
(b) 37, 19, 3, 55
(c) 9, 17, 35,
53
(d) 12, 20, 56, 88
Answer
Answer: (b) 37, 19, 3, 55
30.
The element with atomic number 14 is hard and forms acidic
oxide and a covalent halide. To which of the following categories does the
element belong?
(a) Metal
(b) Metalloid
(c) Non-metal
(d) Left-hand
side element
Answer
Answer: (b) Metalloid
31.
The amount of energy released when one or more electrons is
added to the neutral atom is
(a) electron affinity
(b) ionisation
energy
(c) electron negativity
(d) atomicity
Answer
Answer: (a) electron affinity
32.
The valence shell of element A contains 3 electrons while the
valence shell of element B contains 6 electrons. If A combines with B, the
probable chemical formula of the compound is
(a) AB2
(b)
A2B
(c) A2B3
(d)
A3B2
Answer
Answer: (c) A2B3
33.
Arrange the following elements in the order of their
increasing non-metallic character.
Li, O, C, Be, F
(a) F < O < C
< Be < Li
(b) Li < Be < C < O < F
(c) F < O < C
< Be < Li
(d) F < O < Be < C <
Li
Answer
Answer: (b) Li < Be < C < O < F
34.
What type of oxide would E&a-aluminium form?
(a)
EO3
(b) E3O2
(c)
E2O3
(d) EO
Answer
Answer: (c) E2O3
35.
Which of these belong to the same period?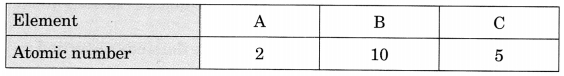
(a) A, B
(b) B, C
(c) C, A
(d) A, B & C
Answer
Answer: (b) B, C
36.
Which of the following property will be common in group 1
elements?
(a) Mass number
(b) Number of protons in nucleus
(c) Atomic
number
(d) Number of valence electrons
Answer
Answer: (d) Number of valence electrons
37.
Which one of the following elements exhibit maximum number of
valence electrons?
(a) Na
(b) Al
(c) Si
(d)
P
Answer
Answer: (d) P
38.
Which of the following gives the correct increasing order of
the atomic radii of O, F and N?
(a) O, F, N
(b) N, F, O
(c) O, N, F
(d) F, O, N
Answer
Answer: (d) F, O, N
39.
Element ‘A’ has electronic configuration 2, 7; ‘B’ has
electronic configuration 2, 8, 5 and ‘C’ has electronic configuration 2, 8, 7.
Which two elements will exhibit similar chemical properties?
(a) A and C
(b) A and B
(c) B and C
(d) None of these
Answer
Answer: (a) A and C
40.
Which of the following elements does not lose an electron
easily?
(a) Na
(b) F
(c) Mg
(d) Al
Answer
Answer: (b) F
41.
Which of the following are the characteristics of isotopes of
an element?
(i) Isotopes of an element have same atomic masses
(ii)
Isotopes of an element have same atomic number
(iii) Isotopes of an element
show same physical properties
(iv) Isotopes of an element show same chemical
properties
(a) (i), (iii) and (iv)
(b) (ii), (iii) and (iv)
(c) (ii)
and (iii)
(d) (ii) and (iv)
Answer
Answer: (b) (ii), (iii) and (iv)
42.
An element-X has mass number 40 and contains 21 neutrons in
its atom. To which group of the periodic table does it belong?
(a) Group
1
(b) Group 4
(c) Group 2
(d) Group 3
Answer
Answer: (b) Group 4
43.
Which of the following properties does not match element of
the halogen family?
(a) They have seven electrons in their valence shell.
(b) They are highly reactive.
(c) They are metallic in nature.
(d) They
are diatomic in their molecular form.
Answer
Answer: (c) They are metallic in nature.
44.
Upto which element, the Law of Octaves was found to be
applicable.
(a) Oxygen
(b) Calcium
(c) Cobalt
(d)
Potassium
Answer
Answer: (b) Calcium
45.
According to Medeleev’s Periodic Law, the elements were
arranged in the periodic table in the order of
(a) increasing atomic
number
(b) decreasing atomic number
(c) increasing atomic masses
(d)
decreasing atomic masses
Answer
Answer: (c) increasing atomic masses
46.
Consider the following elements:
20Ca,
8O, 18Ar, 16S, 4Be,
2He
Which of the above elements would you expect to be in group 16
of the periodic table?
(a) 20Ca and 16S
(b)
20Ca and 8O
(c) 18Ar and 16S
(d) 8O and 16S
Answer
Answer: (d) 8O and 16S
47.
The electronic configuration of the atom of an element X is
2, 4, 8. In the modern periodic table, the element X is placed in:
(a) 2nd
group
(b) 4th group
(c) 14th group
(d) 8th
group
Answer
Answer: (c) 14th group
48.
Which of the following element would lose an electron
easily?
(a) Mg
(b) Na
(c) K
(d) Ca
Answer
Answer: (c) K
49.
The element A, B, C, D and E have atomic numbers 9, 11, 17,
12 and 13 respectively. The pair of elements which belongs to the same group of
the periodic table is:
(a) A and B
(b) B and D
(c) A and C
(d) D and
E
Answer
Answer: (c) A and C
50.
Which one of the following does not increase while moving
down the group of the periodic table?
(a) Atomic radius
(b) Metallic
character
(c) Valence electrons
(d) Shells in the
atoms
Answer
Answer: (c) Valence electrons
51.
The Newlands Law of Octaves for the classification of
elements was found to be applicable only up to the element
(a) Potassium
(b) Calcium
(c) Cobalt
(d) Thorium
Answer
Answer: (b) Calcium
52.
An element X forms an oxide X^g. In which group of Modem
periodic table is this element placed?
(a) Group 2
(b) Group 3
(c)
Group 5
(d) Group 13
Answer
Answer: (b) Group 3
53.
The atomic numbers of the elements Na, Mg, K and Ca are 11,
12, 19 and 20 respectively. The element have the largest atomic radius is:
(a) Mg
(b) Na
(c) K
(d) Ca
Answer
Answer: (c) K
54.
Where would you locate the element with electronic
configuration 2, 8 in the Modern Periodic Table?
(a) Group 8
(b) Group
18
(c) Group 10
(d) Group 16
Answer
Answer: (b) Group 18
55.
Which of the following is the valance shell for the elements
of second period of the modern periodic table?
(a) M shell
(b) K shell
(c) L shell
(d) N shell
Answer
Answer: (c) L shell
56.
Which of the following statements is not a correct statement
about the trends when going from left to right across the periods of the
periodic table?
(а) The elements becomes less metallic in nature
(b) The
number of valance electrons increases
(c) The atoms lose their electrons more
easily
(d) The oxides become more acidic
Answer
Answer: (c) The atoms lose their electrons more easily
57.
The longest period of the Modern Periodic Table is
period.
(a) first
(b) second
(c) fourth
(d)
sixth
Answer
Answer: (d) sixth
58.
The inert gas which lies in third period is
(a) Helium
(b) Neon
(c) Argon
(d) Xenon
Answer
Answer: (c) Argon
59.
An element with atomic number 40 is placed in period.
(a)
5
(b) 6
(c) 4
(d) 3
Answer
Answer: (a) 5
60.
The total number of electrons present in the first twenty
elements of the periodic table is
(a) 250
(b) 230
(c) 210
(d)
190
Answer
Answer: (c) 210
61.
The element with atomic number 26 will be found in group
(a) 2
(b) 8
(c) 6
(d) 10
Answer
Answer: (b) 8
62.
Eka-aluminium and Eka-silicon are known as
(a) gallium and
germanium
(b) aluminium and silicon
(c) iron and sulphur
(d) neutron
and proton
Answer
Answer: (a) gallium and germanium
63.
When an atom of iodine becomes an iodine ion (I–)
the radius will
(a) decrease
(b) increase
(c) remain the same
(d)
none
Answer
Answer: (b) increase
64.
Which type of elements are poor conductors of heat and
electricity?
(a) Metals
(b) Non-metals
(c) Gases
(d) None of
these
Answer
Answer: (b) Non-metals
65.
What is the symbol of the element Tungsten?
(a) Tu
(b)
W
(c) Ta
(d) Tw
Answer
Answer: (b) W
66.
Which of the element whose atomic number is given below
cannot accommodate in the present set up of the long form of the periodic
table?
(a) 107
(b) 118
(c) 102
(d) 126
Answer
Answer: (d) 126