Carbon and its Compounds
Class 10th Science Chapter MCQs
Carbon and Its Compounds Class 10 MCQs Questions
1. Which of the following statements are correct for carbon compounds?
(i)
Most carbon compounds are good conductors of electricity.
(ii) Most carbon
compounds are poor conductors of electricity.
(iii) Force of attraction
between molecules of carbon compounds is not very strong.
(iv) Force of
attraction between molecules of carbon compounds is very strong.
(a) (ii) and
(iv)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (i) and
(iii)
Answer
Answer: b
2. C3H8 belongs to the homologous series of
(a)
Alkynes
(b) Alkenes
(c) Alkanes
(d) Cyclo
alkanes
Answer
Answer: c
3.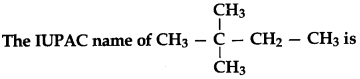
(a) 2-ethyl-2-methyl propane
(b) 2, 2-demethyl butane
(c) 1,1,1-trimethyl propane
(d) 2, 2-methyl butane
Answer
Answer: b
4. Which of the following is the formula of Butanoic acid?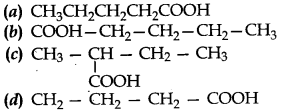
Answer
Answer: d
5. The number of isomers of pentane is
(a) 2
(b) 3
(c) 4
(d)
5
Answer
Answer: b
6. Which of the following will undergo addition reactions?
(a)
CH4
(b) C3H8
(C)
C2H6
(d)
C2H4
Answer
Answer: d
7. When ethanoic acid is treated with NaHCO^ the gas evolved is
(a)
H2
(b) CO2
(c) CH4
(d)
CO
Answer
Answer: b
8. Ethanol on complete oxidation gives
(a) acetic acid/ethanoic acid
(b) CO2 and water
(c) ethanal
(d)
acetone/ethanone
Answer
Answer: b
9. Which of the following will give a pleasant smell of ester when heated
with ethanol and a small quantity of sulphuric acid?
(a)
CH3COOH
(b) CH3CH2OH
(c)
CH3OH
(d) CH3CHO
Answer
Answer: a
10. Name the functional group present in CH3COCH3.
(a) Alcohol
(b) Carboxylic acid
(c) Ketone
(d)
Aldehyde
Answer
Answer: c
11. Why does carbon form compounds mainly by covalent bonding?
(a) There
are four electrons in the outermost shell of carbon.
(b) It requires large
amount of energy to form C4+ or C4sup>4-.
(c) It shares its
valence electrons to complete its octet.
(d) All the
above.
Answer
Answer: d
12. Addition reactions are undergone by
(a) saturated hydrocarbons
(alkanes)
(b) only alkenes
(c) only alkynes
(d) both alkenes and
alkynes
Answer
Answer: d
13. Identify ‘A’ in the following reaction:
CH3COOH +
Na2CO3 → A + CO2 + H0O
(a)
CH3COONa
(b) CH2(Na)COOH
(c) NaOH
(d)
NaHCO3
Answer
Answer: a
14. Which of the following belongs to homologous series of alkynes?
C6H6, C2H6,
C2H4, C3H4.
(a)
C6H6
(b) C2H4
(C)
C2H6
(d)
C3H4
Answer
Answer: d
15. A hydrocarbon has four carbon atoms. Give its molecular formula if it is
an alkene.
(a) C4H10
(b)
C4H8
(C) C4H6
(d)
C4H4
Answer
Answer: b
16. Identify A and B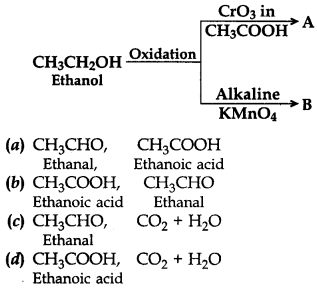
Answer
Answer: a
17. Give the IUPAC name of CH3COOC2H5.
(a) Ethyl ethanoic acid
(b) Butanoate
(c) Ethyl ethanoate
(d) Etyl
methyl carboxylic acid
Answer
Answer: c
18. The firSt member of the alkyne homologous series is
(a) propyne
(b)
ethyne
(c) methane
(d) ethene
Answer
Answer: b
19. In diamond, each carbon atom is bonded to four other carbon atoms to
form
(a) a hexagonal array
(b) a rigid three-dimensional structure
(c)
a structure in the shape of a football
(d) a structure of a
ring
Answer
Answer: b
20. A soap molecule has a
(a) hydrophobic head and hydrophobic tail
(b)
hydrophobic head and hydrophilic tail
(c) hydrophilic head and hydrophilic
tail
(d) hydrophilic head and hydrophobic
tail
Answer
Answer: d
21.
Carbon exists in the atmosphere in the form of
(a) carbon
monoxide only
(b) carbon monoxide in traces and carbon dioxide
(c) carbon
dioxide only
(d) coal
Answer
Answer: (b) carbon monoxide in traces and carbon dioxide
22.
Graphite is used as a lubricant in machines because
(a) it
is a good conductor of electricity.
(b) it has a high melting point and
slippery layers.
(c) its density ranges from 1.9 to 2.3 g/cm3.
(d) it is strong and soft.
Answer
Answer: (b) it has a high melting point and slippery layers.
23.
The allotrope of carbon which is a good conductor of heat and
electricity is
(a) diamond
(b) graphite
(c) charcoal
(d) none of
these
Answer
Answer: (b) graphite
24.
Which of the following does not belong to the same homologous
series?
(a) CH4
(b) C2H6
(c)
C3H8
(d)
C4H8
Answer
Answer: (d) C4H8
25.
Which of the following is not considered as crystalline
allotrope of carbon?
(a) Coal
(b) Diamond
(c) Graphite
(d)
Fullerence
Answer
Answer: (a) Coal
26.
Which form of carbon is found in Golkonda mines of
Karnataka?
(a) Diamond
(b) Graphite
(c) Coal
(d)
Coke
Answer
Answer: (a) Diamond
27.
Lead pencil contains
(a) graphite
(b) diamond
(c)
lead
(d) lead sulphate
Answer
Answer: (a) graphite
28.
Mineral acids are stronger acids than carboxylic acids
because
(i) mineral acids are completely ionised
(ii) carboxylic acids are
completely ionised
(iii) mineral acids are partially ionised
(iv)
carboxylic acids are partially ionised
(a) (i) and (iv)
(b) (ii) and
(iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer
Answer: (a) (i) and (iv)
29.
When ethyl alcohol and acetic acid are mixed, the resulting
ester has a chemical formula
(a)
CH3COOC2H5
(b)
C2H5COOCH3
(c)
C2H5COOC2H5
(d)CH3COOCH3
Answer
Answer: (a) CH3COOC2H5
30.
A few drops of ethanoic acid were added to solid sodium
carbonate. The observation made was that
(a) a hissing sound was produced
(b) brown fumes evolved
(c) brisk effervescence occurred
(d) a pungent
smelling gas evolved
Answer
Answer: (c) brisk effervescence occurred
31.
A reagent which can help us to distinguish between alkenes
and alkynes is:
(a) Bromine water
(b) Carbon tetrachloride
(c) Alkaline
KMnO4
(d) Ammoniacal cuprous chloride
Answer
Answer: (d) Ammoniacal cuprous chloride
32.
The correct structural formula of butanoic acid is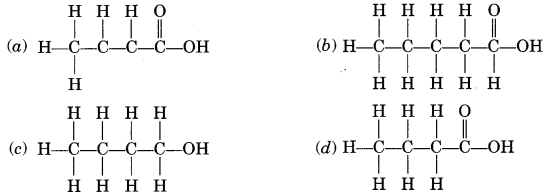
Answer
Answer: (d)
33.
The soap molecule has a
(a) hydrophilic head and a
hydrophobic tail
(b) hydrophobic head and a hydrophilic tail
(c)
hydrophobic head and a hydrophobic tail
(d) hydrophilic head and a
hydrophilic tail
Answer
Answer: (a) hydrophilic head and a hydrophobic tail
34.
Detergents can be used for washing of clothes
(a) only in
hard water
(b) only in soft water
(c) both in soft and hard water
(d)
None of the above
Answer
Answer: (c) both in soft and hard water
35.
Which among the following are unsaturated
hydrocarbons?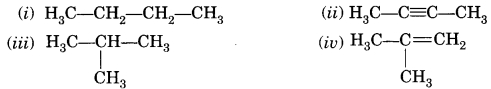
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and
(iv)
Answer
Answer: (c) (ii) and (iv)
36.
Acetic acid was added to a solid X kept in a test tube. A
colourless, odourless gas Y was evolved. The gas was passed through lime water,
which turned milky. It was concluded that
(a) solid X is sodium hydroxide and
the gas Y is CO2
(b) solid X is sodium bicarbonate and the gas Y
is CO2
(c) solid X is sodium acetate and the gas Y is
CO2
(d) solid X is sodium bicarbonate and the gas Y is
SO2.
Answer
Answer: (b) solid X is sodium bicarbonate and the gas Y is CO2
37.
Structural formula of ethyne is
Answer
Answer: (a)
38.
A student while observing the properties of acetic acid would
report that this smells like
(a) vinegar and turns red litmus blue
(b)
rotten egg and turns red litmus blue
(c) vinegar and turns blue litmus
red
(d) rotten egg and turns blue litmus red
Answer
Answer: (c) vinegar and turns blue litmus red
39.![]()
In the above given reaction, alkaline KMnO4 acts as
(a) reducing
agent
(b) oxidising agent
(c) catalyst
(d) dehydrating
agent
Answer
Answer: (b) oxidising agent
40.
Vinegar is a solution of
(a) 50% – 60% acetic acid in
alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in
water
(d) 50% – 60% acetic acid in water
Answer
Answer: (c) 5% – 8% acetic acid in water
41.
The portion left on dropping a hydrogen atom from an alkane
is called
(a) functional group
(b) alkenyl group
(c) alkyl group
(d)
phenyl group
Answer
Answer: (c) alkyl group
42.
The functional group present in a carboxylic acid is
Answer
Answer: (b)
43.
Identify the unsaturated compounds from the following:
(i)
Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and
(ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (ii) and
(iii)
Answer
Answer: (d) (ii) and (iii)
44.
Which of the following are correct structural isomers of
butane?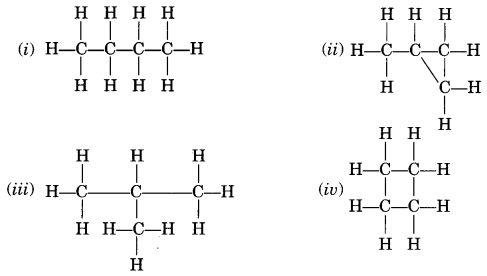
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and
(iv)
Answer
Answer: (c) (i) and (ii)
45.
Carbon forms four covalent bonds by sharing its four valence
electrons with four univalent atoms, e.g., hydrogen. After the formation of four
bonds, carbon attains the electronic configuration of
(a) helium
(b)
neon
(c) argon
(d) krypton
Answer
Answer: (b) neon
46.
Which of the following statements are usually correct for
carbon compounds? These
(i) are good conductors of electricity
(ii) are
poor conductors of electricity
(iii) have strong forces of attraction between
their molecules
(iv) do not have strong forces of attraction between their
molecules
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d)
(ii) and (iv)
Answer
Answer: (d) (ii) and (iv)
47.
Pentane has the molecular formula
C5H12. It has
(a) 5 covalent bonds
(b) 12 covalent
bonds
(c) 16 covalent bonds
(d) 17 covalent
bonds
Answer
Answer: (c) 16 covalent bonds
48.
How many electrons are there in the outermost orbit of
carbon?
(a) Two
(b) Three
(c) One
(d)
Four
Answer
Answer: (d) Four
49.
Which of these is true for most of the organic compounds?
(a) High melting and boiling points
(b) Low melting point but high boiling
point
(c) High melting point but low boiling point
(d) Low melting and
boiling points
Answer
Answer: (b) Low melting point but high boiling point
50.
Hydrocarbons are mainly composed of which of these?
(a)
Hydrogen, carbon and nitrogen
(b) Hydrogen and carbon
(c) Hydrogen
(d)
Hydrogen, oxygen and carbon
Answer
Answer: (b) Hydrogen and carbon
51.
How many double bonds are there in a saturated
hydrocarbon?
(a) One
(b) Two
(c) Three
(d)
Zero
Answer
Answer: (d) Zero
52.
Successive members of a homologous series vary by how many
atomic mass unit?
(a) Sixteen
(b) Fourteen
(c) One
(d)
Twelve
Answer
Answer: (b) Fourteen
53.
Ethanol is also known as which of these?
(a) Formic
acid
(b) Ethyl alcohol
(c) Ethane
(d)
Acetaldehyde
Answer
Answer: (b) Ethyl alcohol
54.
Ethanoic acid is also known as which of these?
(a) Citric
acid
(b) Nitric acid
(c) Acetic acid
(d) Formic
acid
Answer
Answer: (c) Acetic acid
55.
Soaps are ester of which type of acids?
(a) Formic
acid
(b) Fatty acid
(c) Inorganic acid
(d) Acetic
acid
Answer
Answer: (b) Fatty acid
56.
Which of these is not an organic acid?
(a) Acetic acid
(b) Tartaric acid
(c) Nitric acid
(d) Formic
acid
Answer
Answer: (c) Nitric acid
57.
Which of the following is not an allotropic form of
carbon?
(a) Fluorine
(b) Fullerene
(c) Diamond
(d)
Graphite
Answer
Answer: (a) Fluorine