Metals and Non-metals
Class 10th Science Chapter MCQs
Metals and Non-metals Class 10 MCQs Questions
1.
The ability of metals to be drawn into thin wires is known
as
(a) ductility
(b) malleability
(c) sonority
(d)
conductivity
Answer
Answer: (a) ductility
2.
Aluminium is used for making cooking utensils. Which of the
following properties of aluminium are responsible for the same?
(i) Good
thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and
(iii)
(d) (i) and (iv)
Answer
Answer: (d) (i) and (iv)
3.
Due to its semiconductor properties the non-metal used in
computer, T.V. etc. is
(a) Carbon
(b) Silicon
(c) Bromine
(d)
Fluorine
Answer
Answer: (b) Silicon
4. Aluminium is used for making cooking uten¬sils. Which of the following
properties of alu¬minium are responsible for the same?
(i) Good thermal
conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv)
High melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and
(iii)
(d) (i) and (iv)
Answer
Answer: d
5. The most abundant metal in the earth’s crust is
(a) Iron
(b)
Aluminium
(c) Calcium
(d) Sodium
Answer
Answer: b
6. The poorest conductor of heat among metals is
(a) Lead
(b)
Mercury
(c) Calcium
(d) Sodium
Answer
Answer: a
7. Which property of metals is used for making bells and strings of musical
instruments like Sitar and Violin?
(a) Sonorousness
(b) Malleability
(c) Ductility
(d) Conductivity
Answer
Answer: a
8. Al2O3 + 2NaOH → …… + H2O
(a)
Al(OH)3
(b) Na2O
(c) NaAlO2
(d)
AlNaO2
Answer
Answer: c
9. Which of the following is the correct arrange-ment of the given metals in
ascending order of their reactivity?
Zinc, Iron, Magnesium, Sodium
(a)
Zinc > Iron > Magnesium > Sodium
(b) Sodium > Magnesium > Iron
> Zinc
(c) Sodium > Zinc > Magnesium > Iron
(d) Sodium >
Magnesium > Zinc > Iron
Answer
Answer: d
10. Which of the following pairs will give dis-placement reactions?
(a)
FeSO4 solution and Copper metal
(b) AgNO3 solution and
Copper metal
(c) CuSO4 solution and Silver metal
(d) NaCl
solution and Copper metal
Answer
Answer: b
11. Non-metals form covalent chlorides because
(a) they can give electrons
to chlorine
(b) they can share electrons with chlorine
(c) they can give
electrons to chlorine atoms to form chloride ions
(d) they cannot share
electrons with chlorine atoms
Answer
Answer: b
12. Which of the following oxide(s) of iron would be obtained on prolonged
reaction of iron with steam?
(a) FeO
(b) Fe2O3
(c) Fe3O4
(d) Fe2O3 and
Fe2O4
Answer/ Explanation
Answer: c
Explaination: Reason: 3Fe (s) + 4H2O (g) →
Fe3O4 (s) + 4H2 (g)
13. Which of tire following are not ionic compounds?
(i) KCl
(ii)
HCl
(iii) CCl4
(iv) NaCl
(a) (i) and (ii)
(b) (ii) and
(iii)
(c) (iii) and (iv)
(d) (i) and
(iii)
Answer
Answer: b
14. The electronic configuration of three elements X, Y and Z are as
follows:
X = 2, 4, Y = 2, 7, Z = 2,1 Which two elements will combine to form
an ionic compound and write the correct formula,
(a) X2Y
(b)
YZ
(c) XZ3</sub
(d)
Y2Z
Answer
Answer: b
15. The highly reactive metals like Sodium, Potas-sium, Magnesium, etc. are
extracted by the
(a) electrolysis of their molten chloride
(b)
electrolysis of their molten oxides
(c) reduction by aluminium
(d)
reduction by carbon
Answer
Answer: a
16. Which of the following non-metal is lustrous?
(a) Sulphur
(b)
Oxygen
(c) Nitrogen
(d) Iodine
Answer
Answer: d
17. Example of an amphoteric oxide is:
(a) Na2O
(b)
K2O
(C) Al2O3
(d)
MgO
Answer
Answer: c
18. Which one among the following is an acidic oxide?
(a)
Na2O
(b) CO
(c) CO2
(d)
Al2O3
Answer
Answer: c
19. The atomic number of an element ‘X’ is 12. Which inert gas is nearest to
X?
(a) He
(b) Ar
(c) Ne
(d) Kr
Answer/ Explanation
Answer: c
Explaination: Reason: ‘X’ is Magnesium and Argon (Ar) with
atomic number 12 is the closest inert gas to it.
20. The process in which a carbonate ore is heated strongly in the absence of
air to convert it into metal oxide is called
(a) Roasting
(b)
Reduction
(c) Calcination
(d) Smelting
Answer
Answer: c
21. Oxides of moderately reactive metals like Zinc, Iron, Nickel, Tin, Copper
etc. are reduced by using
(a) Aluminium as reducing agent
(b) Sodium as
reducing agent
(c) Carbon as reducing agent
(d) Calcium as reducing
agent
Answer
Answer: c
22. In thermite welding a mixture of …… and …… is ignited with a burning
magnesium ribbon which produces molten iron metal as large amount of heat is
evolved.
(a) iron (III) oxide and aluminium powder
(b) iron (II) oxide and
aluminium powder
(c) iron (III) chloride and aluminium powder
(d) iron
(III) sulphate and aluminium powder
Answer
Answer: a
23. Galvanisation is a method of protecting iron from rudftng by coating with
a thin layer of
(a) Galium
(b) Aluminium
(c) Zinc
(d)
Silver
Answer
Answer: c
24. An element X is soft and can be cut with a knife. This is very reactive
to air and cannot be kept open in air. It reacts vigorously with water. Identify
the element from the following
(a) Mg
(b) Na
(c) P
(d)
Ca
Answer
Answer: b
25. Reaction between X and Y forms compound Z. X loses electron and Y gains
electron. Which of the following properties is not shown by Z?
(a) Has high
melting point
(b) Has low melting point
(c) Conducts electricity in molten
state
(d) Occurs as solid
Answer/ Explanation
Answer: c
Explaination: Reason: Z is an ionic compound. It has a high
melting point.
26. The electronic configurations of three ele¬ments X, Y and Z are X — 2, 8;
Y — 2, 8, 7 and Z — 2, 8, 2. Which’of the following is correct?
(a) X is a
metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z
is a metal
Answer/ Explanation
Answer: c
Explaination: Reason: According to the electronic configuration,
Y is Chlorine and Z is Magnesium.
27. Amalgam is an alloy of
(a) Copper and Tin
(b) Mercury
(c) Lead
and Tin
(d) Copper and Zinc
Answer
Answer: b
28. Copper objects lose their shine and form green coating of
(a) Copper
oxide
(b) Copper hydroxide and Copper oxide
(c) Basic Copper carbonate
(d) Copper carbonate
Answer
Answer: c
29.
What happens when calcium is treated with water?
(i) It
does not react with water.
(ii) It reacts violently with water.
(iii) It
reacts less violently with water.
(iv) Bubbles of hydrogen gas formed stick
to the surface of calcium.
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i)
and (ii)
(d) (iii) and (iv)
Answer
Answer: (d) (iii) and (iv)
30.
Generally metals react with acids to give salt and hydrogen
gas. Which of the following acids does not give hydrogen gas on reacting with
metals (except Mn and Mg)?
(a) H2SO4
(b) HCl
(c)
HNO3
(d) All of these
Answer
Answer: (c) HNO3
31.
Which of the following metals are obtained by electrolysis of
their chlorides in molten state?
(i) Na
(ii) Ca
(iii) Fe
(iv) Cu
(a) (i) and (iv)
(b) (iii) and (iv)
(c) (i) and (iii)
(d) (i) and
(ii)
Answer
Answer: (d) (i) and (ii)
32.
An alloy reacted with dilute hydrochloric acid to produce a
gas which ‘pops’ a lighted splint. The residue reacted with dilute nitric acid
to form a blue solution. Which one of the following pairs of metals is present
in the alloy?
(a) Copper and lead
(b) Lead and magnesium
(c) Copper and
magnesium
(d) Lead and zinc
Answer
Answer: (c) Copper and magnesium
33.
Which of the following metals exist in their native state in
nature?
(i) Cu
(ii) Au
(iii) Zn
(iv) Ag
(a) (i) and (ii)
(b)
(ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and
(iv)
Answer
Answer: (c) (ii) and (iv)
34.
Metals are refined by using different methods. Which of the
following metals are refined by electrolytic refining?
(i) Au
(ii) Cu
(iii) Na
(iv) K
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and
(iii)
(d) (iii)and (iv)
Answer
Answer: (a) (i) and (ii)
35.
Metal M occurs in the Earth’s crust as its oxide
M2O3. An alloy of this metal is used in making aircrafts.
The ore of the metal M is
(a) magnetite
(b) haematite
(c) bauxite
(d) pyrolusite
Answer
Answer: (c) bauxite
36.
Which one of the following four metals would be displaced
from the solution of its salts by other three metals?
(a) Mg
(b) Ag
(c)
Zn
(d) Cu
Answer
Answer: (b) Ag
37.
2 mL each of concentrated HCl, HNO3 and a mixture
of concentrated HCl and concentrated HNO3 in the ratio of 3 : 1 were
taken in test tubes labelled as A, B and C. A small piece of metal was put in
each test tube. No change occurred in test tubes A and B but the metal got
dissolved in test tube C respectively. The metal could be
(a) Al
(b)
Au
(c) Cu
(d) Pt
Answer
Answer: (b) Au
38.
Which one of the following figures correctly describes the
process of electrolytic refining?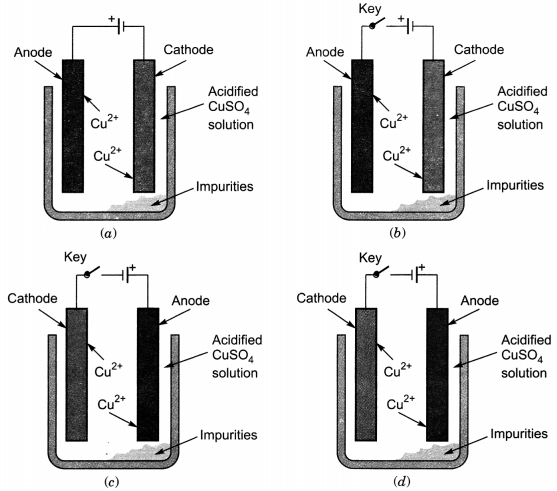
Answer
Answer: (c)
39.
An element A is soft and can be cut with a knife. This is
very reactive to air and cannot be kept in open. It reacts vigorously with
water. Identify the element from the following:
(a) Mg
(b) Na
(c) P
(d) Ca
Answer
Answer: (b) Na
40.
Alloys are homogeneous mixtures of a metal with a metal or
non-metal. Which among the following alloys contain non-metal as one of its
constituents?
(a) Brass
(b) Bronze
(c) Amalgam
(d)
Steel
Answer
Answer: (d) Steel
41.
Reaction between X and Y, forms compound Z. X loses electron
and Y gains electron. Which of the following properties is not shown by Z?
(a) Has high melting point
(b) Has low melting point
(c) Conducts
electricity in molten state
(d) Occurs as solid
Answer
Answer: (b) Has low melting point
42.
The electronic configurations of three elements X, Y and Z
are X – 2, 8; Y – 2, 8, 7 and Z – 2, 8, 2. Which of the following is
correct?
(a) X is a metal.
(b) Y is a metal.
(c) Z is a non-metal.
(d) Y is a non-metal and Z is a metal.
Answer
Answer: (d) Y is a non-metal and Z is a metal.
43.
Generally, non-metals are not conductors of electricity.
Which of the following is a good conductor of electricity?
(a) Diamond
(b)
Graphite
(c) Sulphur
(d) Fullerene
Answer
Answer: (b) Graphite
44.
Which of the following can undergo a chemical reaction?
(a) MgSO4 + Fe
(b) ZnSO2 + Fe
(c) MgSO2 +
Pb
(d) CuSO2 + Fe
Answer
Answer: (d) CuSO2 + Fe
45.
The atomic numbers of four elements A, B, C and D are 6, 8,
10 and 12 respectively. The two elements which can react to form ionic bonds (or
ionic compound) are:
(a) A and D
(b) B and C
(c) A and C
(d) B and
D
Answer
Answer: (d) B and D
46.
The atomic number of an element X is 19. The number of
electrons in its ion X+ will be:
(a) 18
(b) 19
(c) 20
(d)
21
Answer
Answer: (a) 18
47.
The atomic number of an element Y is 17. The number of
electrons in its ion Y– will be:
(a) 17
(b) 18
(c) 19
(d)
20
Answer
Answer: (b) 18
48.
Which of the following is an iron ore?
(a) Cinnabar
(b)
Calamine
(c) Haematite
(d) Rock salt
Answer
Answer: (c) Haematite
49.
The metal which can be extracted from the bauxite ore is:
(a) Na
(b) Mn
(c) Al
(d) Hg
Answer
Answer: (c) Al
50.
In stainless steel alloy, iron metal is mixed with:
(a) Cu
and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and
Ni
Answer
Answer: (b) Cr and Ni
51.
Which of the following is an ore of mercury metal?
(a)
Rock salt
(b) Cinnabar
(c) Calamine
(d)
Haematite
Answer
Answer: (b) Cinnabar
52.
Which of the following pair of metals exist in their native
state in nature?
(a) Ag and Hg
(b) Ag and Zn
(c) Au and Hg
(d) Au
and Ag
Answer
Answer: (d) Au and Ag
53.
Which of the following alloys contains a non-metal as one of
the constituents?
(a) Brass
(b) Amalgam
(c) Steel
(d)
Bronze
Answer
Answer: (c) Steel
54.
The metal which is always present in an amalgam is:
(a)
iron
(b) aluminium
(c) mercury
(d) magnes
Answer
Answer: (c) mercury
55.
Rock salt is an ore of one of the following metals. This
metal is:
(a) Mn
(b) Na
(c) Fe
(d) Cu
Answer
Answer: (b) Na