Metals and Non-metals
Class 10th Science Chapter HOTs
HOTS Questions for Class 10 Science Chapter 3 Metals and Non-metals
Question
1.
A student has been collecting silver coins and copper
coins. One day she observed a black coating on silver coins and a green coating
on copper coins. Which chemical phenomenon is responsible for these coatings ?
Write the chemical names of black and green coatings ?
Answer:
The phenomenon is known as corrosion. Air contains traces of hydrogen sulphide
gas which reacts with silver metal present in the coin to form silver sulphide.
It is black in colour.
Similarly, copper present in the coin reacts with oxygen and traces of both
carbon dioxide and water vapours present in air to form a green mass. It is
chemically basic copper carbonate :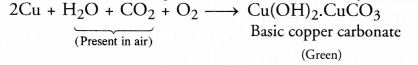
Question
2.
You are provided with three metals : Sodium, magnesium and
copper. Using only water as the reactant, how will you identify them ?
Answer:
The metal
which reacts violently with cold water and catches fire is sodium.
- The metal which evolves hydrogen gas upon heating with water is magnesium.
- The metal which does not react with water even on strong heating is copper.
Question
3.
E is an element amongst copper, zinc, aluminium and iron.
It shows following properties :
(a) One of its ores is rich in
E2O3
(b) E2O3 is not attacked by
water,
(c) It forms two chlorides ECl2 and ECl3. Name
the element and justify your answer.
Answer:
The clue for
the correct answer is the formation of ECl2 and ECl3. This
shows that the element E has variable valencies of 2 and 3. Out of the elements
listed, only iron exists in divalent and trivalent forms.
(a) The ore rich in
Fe2O3 is haematite.
(b) Haematite
(Fe2O3) is not attacked by water.
(c) The two chlorides
are : iron (II) chloride or FeCl2 and iron (III) chloride or
FeCl3.
Question
4.
An element reacts with oxygen to form an oxide which
dissolves in dilute hydrochloric acid. The oxide formed also turns a solution of
red litmus blue. Is the element a metal or non-metal ? Explain with the help of
a suitable example.
Answer:
The oxide of
the element is basic as it turns red litmus solution blue. This means that the
element is a metal (M). Let the metal be sodium (Na). The chemical equations
that are involved are given as follows :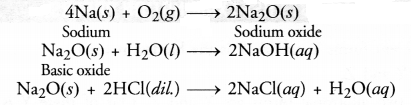
Question
5.
An element E combines with oxygen to form an oxide
E2O which is a good conductor of electricity.
Give the following
information:
- How many electrons will be present in the valence shell of the element E ?
- Write the formula of the compound formed when the element E combines with chlorine.
Answer:
- From the formula E2O of the oxide, it is clear that the valency of the element E is one. This means that it has only one electron in the valence shell.
- We know that chlorine is monovalent. Sine the valency of the element E is a also one, the formula of the chloride of the element is ECl.
Question
6.
An element A’ catches fire in water and burns with golden
yellow flame in air. It reacts with another element ‘B’, present in group 17 to
give a product ‘C’. An aqueous solution of product ‘C’ on electrolysis gives a
compound ‘D’ and liberates hydrogen. Identify A, B, C and D.
Answer:
Since the element A’ catches fire in water and burns with golden yellow flame,
it is sodium (Na). The element ‘B’ with atomic number 17 is chlorine (Cl). Both
these combine to form sodium chloride (NaCl) which is designated as ‘C’. Upon
electrolysis, sodium chloride gives sodium hydroxide (D) and evolves hydrogen
along with chlorine.