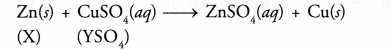Chemical Reactions and Equations
Class 10th Science Chapter HOTs
HOTS Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations
Question
1.
Study the given diagram and answer the following questions
:
(a) Write the chemical reaction involved in the process.
(b) Mention the
colour of :
- copper powder and
- the substance formed after heating it.
(c) How can we reverse the above reaction ? Write the equation for the
reverse reaction and state the substance that undergoes oxidation and the
substance that undergoes reduction.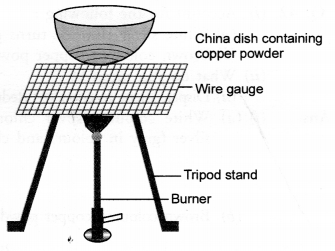
Answer:
(a) Copper
powder upon heating reacts with oxygen (present in air) to form cupric oxide or
copper (II) oxide.![]()
(b)
- Copper powder is brownish in colour.
- The substance formed after heating is cupric oxide. It has black colour.
(c) The reaction can be reversed by heating cupric oxide in a current of
hydrogen.
CuO(s) + H2(g) ———–> Cu (s) + H2O(g)
In this reaction, H2 undergoes oxidation to H2O and CuO is reduced to Cu.
Question
2.
The gases hydrogen and chlorine do not react with each
other even if kept together for a long time. However, in the presence of sun
light, they readily combine. What does actually happen ?
Answer:
We know that in chemical reactions, energy is needed to break the bonds present
in the reacting molecules so that they may combine to form the products. In the
present case, sun light is the source of energy in the form of photons. The
energy made available helps in breaking the bonds present in the reactant
molecules and the chemical reaction leading to hydrogen chloride gas takes
place.![]()
Question
3.
A water insoluble substance ‘X’ on reacting with dilute
H2SO4 released a colourless and
odourless gas accompanied by brisk effervescence. When the gas was passed
through water, the solution obtained turned blue litmus red. On bubbling the gas
through lime water, it initially became milky and the milkiness disappeared when
the gas was passed in excess. Identify the substance ‘X’ and write the chemical
equations of the reactions involved.
Answer:
The water in
soluble substance ‘X’ is most probably some metal carbonate (CaCO3). The chemical equations that
are involved are given.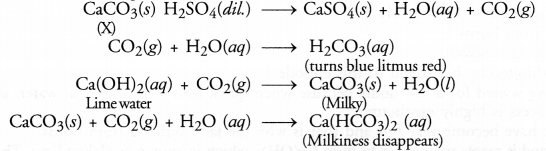
Question
4.
(a) Based on the reactions given below, arrange the metals
involved in these reactions in decreasing order of reactivity. Give suitable
explanation.
- Zn + CuSO4 ———> ZnSO4 + Cu
- Cu + 2AgNO3 ———> Cu(NO3)2 + 2Ag
- Zn + FeSO4 ———-> ZnSO4 + Fe
- Fe + CuSO4 ———-> FeSO4 + Cu
(b) What is the nature of the reactions ?
Answer:
(a)
- Since Zn displaces Cu from CuSO4 solution, it is more reactive than Cu.
- Since Cu displaces Ag from AgNO3 solution, it is more reactive than Ag.
- Since Zn displaces Fe from FeSO4 solution, it is more reactive than Fe.
- Since Fe displaces Cu from CuSO4 solution, it is more reactive
than Cu.
The decreasing order of reactivity of the metals is :
Zn > Fe> Cu> Ag
(b) All these reactions are the examples of displacement reactions.
Question
5.
A, B and C are three elements which undergo chemical
reactions according to following equations.
A2O3 + 2B ———-> B2O3 + 2A
3CSO4 + 2B ———-> B2(SO4)3 + 3C
3CO + 2A ———> A2O3 + 3C
Answer the following
questions :
(a) Which element is the most reactive ?
(b) Which element is
the least reactive ?
Answer:
(a) The most
reactive element is ‘B’ as it has displaced both A’ and ‘C’ from their
compounds.
(b) The least reactive element is ‘C’ as it has been replaced by
both A’ and ‘B’.
Question
6.
You are given the following materials
- Marble chips
- Dilute hydrochloric acid
- Zinc granules
Identify the type of reaction when marble chips and zinc granules are added
separately to acid taken in two tubes. Write chemical equations in each
case.
Answer:
- Marble chips react with dilute hydrochloric acid to form calcium chloride
and carbonic acid. The acid changes to CO2 and H2O. It is a double displacement
reaction.
CaCO3(s) + 2HCl (aq) ———–> CaCl2(aq) + H2CO3(aq) (CO2+H2O) - Zinc granules react with hydrochloric acid to form zinc chloride
accompanied by hydrogen gas. It is a displacement reaction.
Zn(r) + 2HCl(aq) ———–> ZnCl2(aq) + H2(g)
Question
7.
A strip of a metal X is immersed in the aqueous solution
of salt YSO4 blue in colour. After sometime,
a layer the metal Y from the salt solution is deposited on the strip of the
metal X. Whereas the metal X is used for galvanisation, the metal Y is employed
in making electric cables.
- Predict the metal X.
- What could be the metal Y ?
- Can yon name the salt YSO4 ?
- What is the nature of the chemical reaction taking place ?
Answer:
- Since the metal X is used for galvanisation, it is most probably Zn.
- Since the metal Y is used in electric cable, it is likely to be Cu.
- The salt is CuSO4.
- It is an example of metal displacement reaction. Zn lies above Cu in the
activity series and has therefore, displaced Cu from the blue CuSO4 solution.