Science Chapter 5 - Periodic Classification of Elements
Page No 81:
Question 1:-Did Dobereiner’s triads also exist in the columns of
Newlands’ Octaves? Compare and find out.
Answer:
Yes, Dobereiner’s triads also existed in the columns of Newland’s Octaves.
For example, Li, Na, K.
If we consider lithium (Li) as the first element, then sodium (Na) is eighth
element. If we consider sodium as the first element, then potassium is the
eighth element.
Question 2:-What were the limitations of Dobereiner’s classification?
Answer:
It failed to arrange all the then known elements in the form of triads of
elements having similar chemical properties. Dobereiner could identify only
three triads from the elements known that time.
Question 3:-What were the limitations of Newlands’ law of octaves?
Answer:
(i) Newlands law of octaves was applicable to the classification of elements
upto calcium only. After calcium every eighth element did not possess the
properties similar to that of the first element.
(ii) Newlands assumed that only 56 elements existed in nature and no more
elements would be discovered in the future. But later on, several new elements
were discovered whose properties did not fit into Newlands’ law of Octaves.
(iii) In order to fit elements into his table, Newlands put even two elements
together in one slot and that too in the column of unlike elements having very
different properties.
For example, the two elements cobalt (Co) and nickel (Ni) were put together in
just one slot and that too in the column of elements like fluorine, chlorine and
bromine which have very different properties from these elements.
(iv) Iron (Fe) element which resemble elements like cobalt and nickel in
properties, was placed far away from these elements.
Page Number: 85
Question 1:-Use Mendeleev’s Periodic Table to predict the formulae for
the oxides of the following elements: K, C, Al, Si, Ba
Answer:
K2O, CO2,
Al2O3,
SiO2, BaO.
K- K2O
C-C2O4 or
CO2
Al- Al2O3
Si-Si2O4 or
SiO2
Ba2O2 or
BaO
Oxygen is a member of group VI A in Mendeleev’s periodic table. Its
valency is 2. Similarly, the valencies of all the elements listed can be
predicted from their respective groups. This will help in writing the formulae
of their oxides.
(i) Potassium (K) is a member of group IA. Its valency is 1. Therefore,
the formula of it is K2O.
(ii) Carbon (C) is a member of group IV A. Its valency is 4. Therefore,
the formula of it is C2O4 or
CO2.
(iii) Aluminium (Al) belongs to groups III A and its valency is 3. The
formula of its oxide is Al2O3.
(iv) Silicon (Si) is present in group IV A after carbon. Its valency is
also 4. The formula oxide is Si2O4 or
SiO2.
(v) Barium (Ba) belongs to group II A and the valency of the element is 2.
The formula of oxide of the element is Ba2O2 or
BaO.
Question 2:-Besides gallium, which other elements have since been
discovered that were left by Mendeleev in his periodic table? (Any two)
Answer:
Scandium and Germanium.
Question 3:-What were the criteria used by Mendeleev in creating his
Periodic Table?
Answer:
Mendeleev used the relationship between the atomic masses of the elements and
their physical and chemical properties. He used similarity in physical
properties, similarity in the formation of hydrides and oxides of element.
Question 4:-Why do you think the noble gases are placed in a separate
group?
Answer:
Noble gases are chemically inert and are present in atmosphere in extremely low
concentrations. Therefore, owing to their similar inert behavior and similar
electronic configuration, they are justified to be placed in a separate group.
Page Number: 90
Question 1:-How could the modern periodic table remove various
anomalies of Mendeleev’s periodic Table?
Answer:
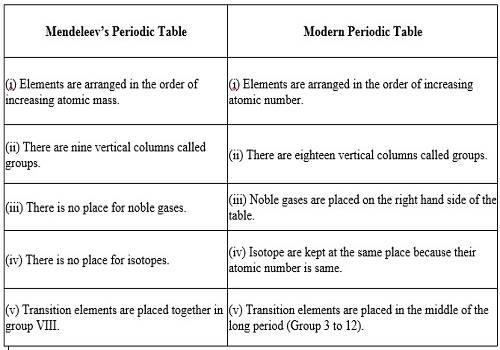
(i) The modern periodic table is based on atomic number, while Mendeleev’s
periodic table was based on atomic mass.
(ii) The isotopes of an element have same number of protons (or atomic number).
So they are alloted the same position in modern periodic table.
(iii) Cobalt and nickel are placed at 9th and 10th position respectively.
(iv) Hydrogen has been alloted special position, i.e., it is placed at the top
of alkali metals in the first group.
Question 2:-Name two elements you would expect to show chemical
reactions similar to magnesium. What is the basis for your choice?
Answer:
Beryllium (Be) and Calcium (Ca).
Both Be (atomic number 4) and Ca (atomic number 20) have similar electronic
configuration, i.e. two electrons in outermost shells.
Be
2,2
Ca 2, 8, 8, 2
Both Be and Ca react with oxygen to give basic oxides, BeO and MgO.
Question 3:-Name :
(a) three elements that have a single electron in their outermost shells.
(b) two elements that have two electrons in their outermost shells.
(c) three elements with filled outer most shells.
Answer:
(a) Lithium : Atomic number – 3(2, 1); Sodium : Atomic number – 11(2, 8, 1);
Potassium : Atomic number – 19(2, 8, 8, 1).
(b) Beryllium : Atomic number – 4(2, 2); Calcium : Atomic number – 20(2, 8, 8,
2)
(c) Helium : Atomic number – 2(2); Neon : Atomic number – 10(2, 8); Argon :
Atomic number – 18(2, 8, 8).
Question 4
(a) Lithium, sodium, potassium are all metals that react with water to liberate
hydrogen gas. Is there any similarity in the atoms of these elements?
(b) Helium is an unreactive gas and neon is a gas of extremely low reactivity.
What, if anything, do their atoms have in common?
Answer:
(a) Lithium, sodium and potassium all belong to the same group. The atoms of
lithium, sodium and potassium all have only one electron in their outermost
shells and all of these are metals. All of these react with water to form
alkalies.
(b) The atoms of helium and neon have their outermost shells completely filled.
Helium has its first shell completely filled, while neon has its first and
second shells (K and L) completely filled.
Question 5:-In the modern periodic table, which are the metals among
the first ten elements?
Answer:
The first ten elements in modern periodic table are hydrogen, helium, lithium,
beryllium, boron, carbon, nitrogen, oxygen, fluorine and neon. Out of these,
lithium, beryllium and boron are metals, because they have 1, 2 and 3 electrons
respectively in their outermost shells.
Question 6:-By considering their position in the Periodic Table, which
one of the following elements would you expect to have maximum metallic
characteristics?
Ga, Ge, As, Se, Be
Answer:
Beryllium (Be). In the periodic table, the elements placed on the left show
maximum metallic characteristics. Since beryllium occupies the most left
position in comparison to other elements, hence it shows maximum metallic
characteristics.
NCERT Solutions for Class 10 Science
Chapter 5
Textbook Chapter End Questions
Question 1:-Which of the following statements is not a correct
statement about the trends when going from left to right across the periods of
Periodic Table.
(a) The elements become less metallic in nature.
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
Answer:
(c) The atoms lose their .electrons more easily.
The atoms lose their electrons more easily.
The atoms lose their electrons more easily is a wrong statement because as
we move from left to right across the periods of the periodic table, the
non-metallic character increases.
Therefore, tendency to lose an electron decreases
Question 2:-Element X forms a chloride with the formula XCl2,
which is solid with a high melting point. X would most likely to be in the same
group of the periodic table as
(a) Na
(b) Mg
(c) Al
(d) Si
Answer:
(b) Mg
Answer is Magnesium because Mg has the valency 2 which is same as the
group (a) Na (b) Mg (c) AI (d) Si
Also, Mg when combines chloride forms MgCl2.
Question 3:-Which element has
(a) two shells, both of which are completely filled with electrons ?
(b) the electronic configuration 2, 8, 2 ?
(c) a total of three shells, with four electrons in its valence shell ?
(d) a total of two shells with three electrons in its valence shell. ?
(e) twice as many electrons in its second shell as in its first shell?
Answer:
(a) Neon (2, 8)
(b) Magnesium
(c) Silicon (2, 8, 4)
(d) Boron (2, 3)
(e) Carbon (2, 4)
a) Neon has two shells which are completely filled.
b) Magnesium has electronic configuration 2, 8, 2
c) Silicon has a total of three shells, with four electrons in its valence
shell
d) Boron a total of two shells, with three electrons in its valence shell
e) Carbon has twice as many electrons in its second shell as in its first
shell
Question 4
(a) What property do all elements in the same column of the Periodic Table as
boron have in common?
(b) What property do all elements in the same column of the Periodic Table. As
fluorine have in common?
Answer:
(a) Elements in the same column or group as boron have valency of three and have
three valence electrons.
(b) Elements in the same column or group as fluorine form acidic oxides and have
seven electrons in their outermost shells and have valency of one.
Question 5:-An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element?
(b) To which of the following elements would it be chemically similar? (Atomic
numbers are given in parentheses.)
N (7), F (9), P (15), Ar (18)
Answer:
(a) The atomic number of the given element is 2 + 8 + 7(= 17).
(b) It would be chemically similar to fluorine [F(9)] because its electronic
configuration is 2, 7.
Question 6:-The positions of three elements A, B and C in the periodic
table are shown below :
(a) State whether A is a metal or non-metal.
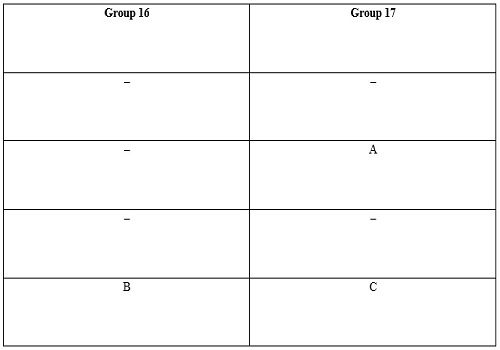
(b) State whether C is more reactive or less reactive than A.
(c) Will C be larger or smaller in size than B ?
(d) Which type of ion, cation or anion, will be formed by element A ?
Answer:
(a) Since the valency of group 17 elements is 1 and all these elements accept
electrons, thus A is a non-metal.
(b) C is less reactive than A because as we move down in a group, the reactivity
of non-metals increases.
(c) C is smaller in size than B because B and C both are related to the same
period and the size decreases as one moves from left to right in a period.
(d) A will form anion because it is a non-metal.
Question 7:-Nitrogen (atomic number 7) and phosphorus (atomic number
15) belong to group 15 of the periodic table. Write the electronic configuration
of these two elements. Which of these will be more electronegative? Why?
Answer:
Electronic configuration of nitrogen -2,5
Electronic configuration of phosphorus = 2, 8, 5
Nitrogen will be more electronegative because outermost shell is nearer to
nucleus and therefore nucleus will attract electrons more strongly. In a group
of the periodic table, electron attracting tendency decreases as we move from
top to bottom.
Question 8:-How does the electronic configuration of an atom relate to
its position in the Modern Periodic Table?
Answer:
Modern periodic table is based on the atomic number and atomic number is
directly related to the electronic configuration. One can find the group number
and period number of an element on the basis of electronic configuration. For
example, if an element has 1 or 2 electrons in its outermost shell, then it
would belong to group 1 or group 2. And if it has 3 or more electrons in its
outermost shell, then it would belong to group 10 4- the number of electrons in
the outermost shell.
All the alkali metals have one electron in their outermost shell, so they
are placed in group 1. Thus, all the group 2 elements have 2 electrons in their
outermost shell. In group 15 elements, there are 5 electrons in their outermost
shell. Similarly, the number of shells in an element indicates its period
number. For example, the atomic number of magnesium is 12 and its electronic
configuration is 2, 8, 2. Thus it is an element of 3rd period.
Question 9:-In the Modern Periodic Table, calcium (atomic number 20) is
surrounded by elements with atomic number 12, 19, 21 and 38. Which of these have
physical and chemical properties resembling calcium?
Answer:
The electronic configuration of elements with :
Atomic number 12 = 2, 8, 2
Atomic number 19 = 2, 8, 8, 1
Atomic number 20 = 2, 8, 8, 2
Atomic number 21 = 2, 8, 9, 2
Atomic number 38 = 2, 8, 18, 8, 2
Elements with atomic number 12 i.e., magnesium (Mg) and 38 i.e., strontium (Sr)
will have similar physical and chemical properties as element with atomic
numbers 20 i.e., calcium (Ca).
Question 10:-Compare and contrast the arrangement of elements in
Mendeleev’s Periodic Table and the Modern Periodic Table.
Answer: