Science Chapter 2 - Acids, Bases and Salts
Page No 18:
Question 1:-You have been provided with three test tubes. One of them
contains distilled water and the other two contain an acidic solution and a
basic solution, respectively. If you are given only red litmus paper, how will
you identify the contents of each test tube?
Answer:
(i) Put the red litmus paper in all the test tubes, turn by turn. The solution
which turns red litmus to blue will be a basic solution. The blue litmus paper
formed here can now be used to test the acidic solution.
(ii) Put the blue litmus paper obtained above in the remaining two test-tubes,
turn-by-turn. The solution which turns the blue litmus paper to red will be the
acidic solution.
(iii) The solution which has no effect on any litmus paper will be neutral and
hence it will be distilled water.
Page Number: 22
Question 1:-Why should curd and sour substances not be kept in brass
and copper vessels?
Answer:
Curd and sour substances should not be kept in brass and copper vessels because
these and other sour food-stuffs contain acids which can react with the metal of
the vessel to form poisonous metal compounds which can cause food poisoning and
affect our health adversely.
Question 2:-Which gas is usually liberated when an acid reacts with a
metal? Illustrate with an example. How will you test for the presence of this
gas?
Answer:
(i) Hydrogen (H2) gas is liberated when an
acid reacts with a metal.
(ii) Illustration: Set up the apparatus as shown in the given figure. Take some
zinc granules in the test tube. Add about 5 mL dilute hydrochloric acid slowly.
Soon the reaction between zinc and hydrochloric acid starts and hydrogen gas is
evolved.
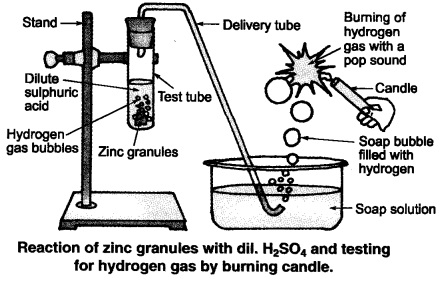
(iii) Test for H2 gas:
H2 gas is not soluble in water. When passed
through soap solution, it gets trapped into bubbles.
Bring a burning candle near the soap bubble filled with gas. The soap bubble
bursts and hydrogen gas burns with a pop sound.
Question 3:-Metal compound A reacts with dilute hydrochloric acid to
produce effervescence. The gas evolved extinguishes a burning candle. Write a
balanced chemical equation for the reaction if one of the compounds formed is
calcium chloride.
Answer:
As the end product is calcium chloride and the gas formed is carbon dioxide, the
metal compound A must be calcium carbonate. Therefore, the reaction between
calcium carbonate and hydrochloric acid is

Page Number: 25
Question 1:-Why do HCl, HNO3, etc show acidic characters in
aqueous solutions while solutions of compounds like alcohol and glucose do not
show acidic character?
Answer:
H+ ions in aqueous solution are responsible
for acidic character. HCl, HNO3, etc. give
H+ ions in water while alcohol and glucose
do not give H+ ion in water. Therefore,
alcohol and glucose do not show acidic character.
Question 2:-Why does an aqueous solution of an acid conduct
electricity?
Answer:
The aqueous solution of an acid conducts electricity due to the presence of
charged particles called ions in it.
Question 3:-Why does dry HCl gas not change the colour of the dry
litmus paper?
Answer:
Dry HCl gas does not give H+ ions and
therefore does not change the colour of dry litmus paper.
Question 4:-While diluting an acid, why is it recommended that the acid
should be added to water and not water to the acid?
Answer:
While diluting an acid it is recommended that the acid should be added to water
and not water to the acid because if water is added to concentrated acid to
dilute it, then a large amount of heat is evolved at once. This heat changes
some of the water to steam explosively which can splash the acid on one’s face
or clothes and cause acid burns.
Question 5:-How is the concentration of hydronium ions (H3O+)
affected when a solution of an acid is diluted?
Answer:
When a given amount of an acid is added to water, there is a fixed number of
hydronium ions per volume of the solution. On dilution, the number of hydronium
ions per volume decreases and concentration decreases.
Question 6:-How is the concentration of hydroxide ions (OH–)
affected when excess base is dissolved in a solution of sodium hydroxide?
Answer:
The concentration of hydroxide ions will increase when excess base is dissolved
in a solution of sodium hydroxide, but it happens to a limited extent only after
which the concentration becomes almost constant.
Page Number: 28
Question 1:-You have two solutions A and B. The pH of solution A is 6
and pH of solution B is 8. Which solution has more hydrogen ion concentration?
Which of this is acidic and which one is basic?
Answer:
A pH value of less than 7 indicates an acidic solution, while greater than 7
indicates a basic solution. Since solution A has more hydrogen ion
concentration, solution A is acidic and solution B is basic.
Question 2:-What effect does the concentration of H+ (aq)
ions have on the nature of the solution?
Answer:
More the concentration of H+ ions, higher
the acidic nature of the solution.
Question 3:-Do basic solutions also have H+ (aq) ions? If
yes, then why are these basic?
Answer:
Basic solutions have H+ (aq) ions. But
these are far less in number than OH– ions
that is responsible for their basic nature.
Question 4:-Under what soil condition do you think a farmer would treat
the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium
hydroxide) or chalk (calcium carbonate)?
Answer:
If the soil is too acidic (having low pH) then it is treated with materials like
quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium
carbonate).
Page Number: 33
Question 1:-What is the common name of the compound CaOCl2 ?
Answer:
Bleaching powder.
Question 2:-Name the substance which on treatment with chlorine yields
bleaching powder.
Answer:
Slaked lime Ca (OH)2.
Question 3:-Name the sodium compound which is used for softening hard
water.
Answer:
Sodium carbonate.
Question 4:-What will happen if a solution of sodium hydrogen carbonate
is heated. Give the equation of the reaction involved?
Answer:
Solution of sodium hydrogen carbonate on heating gives sodium carbonate and
carbon dioxide gas is evolved.
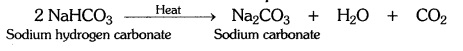
Question 5:-Write an equation to show the reaction between plaster of
Paris and water.
Answer:
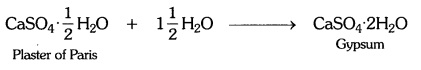
NCERT Solutions for Class 10 Science
Chapter 2
Textbook Chapter End Questions
Question 1:-A solution turns red litmus blue, its pH is likely to be
(a) 1
(b) 4
(c) 5
(d) 10
Answer:
(d) 10
Answer is 10 because litmus paper turns blue when reacts with basic
solution (PH more than 7). Hence 10 is the answer.
Question 2:-A solution reacts with crushed-egg shells to give a gas
that turns lime water milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Answer:
(b) HCl
Egg shells contains calcium carbonate, which on reaction with HCl
liberates CO2 gas which turn lime water to
milky.
CaCO3 + 2HCl → CaCl2 +
H2O + CO2
Question 3:-10 mL of a solution of NaOH is found to be completely
neutralised by 8 mL of a given solution of HC1. If we take 20 mL of the same
solution of NaOH, the amount of HC1 solution (the same solution as before)
required to neutralise it will be
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Answer:
(d) 16 mL
Since 10 ml of NaOH requires 8 mL of HCL, 20 ml of NaOH require 8 x 2 =
16mL of HCl. Hence the answer is option d 16mL.
Question 4:-Which one of the following types of medicines is used for
treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
Answer:
(c) Antacid
Indigestion is due to excess production of acid in the stomach. Medicines
used to treat indigestion is called as Antacid.
Question 5:-Write word equations and then balanced equations for the
reaction taking place when
(a) dilute sulphuric acid reacts with zinc granules
(b) dilute hydrochloric acid reacts with magnesium ribbon
(c) dilute sulphuric acid reacts with aluminium powder
(d) dilute hydrochloric acid reacts with iron filing
Answer:
(a) Zinc + dilute sulphuric acid → Zinc sulphate + Hydrogen
Zn (s) + H2SO4 (aq)
→ ZnSO4 (aq) + H2 (g)
(b) Magnesium ribbon + dil. Hydrochloric acid → Magnesium chloride +
Hydrogen
Mg (s) + 2 HCl (aq) → MgCl2 (aq) + H2 (g)
(c) Aluminium powder + dil. Sulphuric acid > Aluminium sulphate + Hydrogen
2Al (s) + 3H2SO4 (aq)
→ Al2 (SO4)3 (aq)
+ 3H2 (g)
(d) Iron filings + Dilute hydrochloric acid > Ferric chloride + Hydrogen
2Fe (s) + 6HCl (aq) → 2FeCl3 (aq) + 3H2 (g)
Question 6:-Compounds such as alcohol and glucose also contain hydrogen
but are not categorised as acids. Describe an activity to prove it.
Answer:
Though compounds like alcohol and glucose contain hydrogen but they do not
ionise in the solution to produce H+ ions
on passing current through them.
(i) Take solutions of alcohols and glucose.
(ii) Fix two nails on a cork, and place the cork in 100 mL beaker.
(iii) Connect the nails to the two terminals of a 6 volt battery through a bulb
and a switch, as shown in the given Figure.
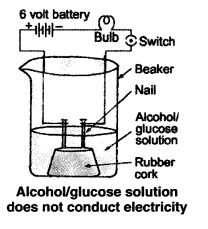
(iv) Now pour alcohol in the beaker and switch on the current.
(v) The bulb does not glow.
(vi) Repeat the experiment with glucose. The bulb does not glow in this case
also.
(vii) This means no ions or H+ ions are present in the solution.
This shows that alcohols and glucose are not acids.
Question 7:-Why does distilled water not conduct electricity, whereas
rainwater does?
Answer:
Distilled water does not conduct electricity because it does not contain any
ionic compound (like acids, bases or salts) dissolved in it.
Rainwater, while falling to the earth through the atmosphere, dissolves an
acidic gas carbon dioxide from the air and forms carbonic acid (H2CO3).
Carbonic acid provides hydrogen ions, H+ (aq)
and carbonate ions, CO(aq)32to
rainwater. Hence, due to the presence of carbonic acid which provides ions to
rainwater, the rainwater conducts electricity.
Question 8:-Why do acids not show acidic behavior in the absence of
water?
Answer:
The acidic behavior of acids is due to the presence of hydrogen ions, [H+ (aq)
ions], in them. The acid produces hydrogen ions only in the presence of water.
So in the absence of water, an acid will not form hydrogen ions and hence will
not show its acidic behavior.
Question 9:-Five solutions A, B, C, D and E when tested with universal
indicator showed pH as 4, 1, 11, 7 and 9 respectively. Which solution is
(a) Neutral
(b) Strongly alkaline
(c) Strongly acidic
(d) Weakly acidic
(e) Weakly alkaline
Arrange the pH in increasing order of hydrogen ion concentration.
Answer:
(a) D
(b) C
(c) B
(d) A
(e) E
Increasing order of hydrogen ion concentration
11 < 9 < 7 < 4 < 1
i. e., C < E < D < A < B
pH 11(C) < pH 9(E) < pH 7 (D) < pH 4 (A) < pH 1 (B)
PH11 – Strongly alkaline
pH9 – weakly alkaline
PH7 – Neutral
pH4 – Weakly acidic
pH1 – Strongly acidic
Question 10:-Equal lengths of magnesium ribbons are taken in test tubes
A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH)
is added to test tube B. In which test tube will the fizzing occur more
vigorously and why?
Answer:
Fizzing will occur more vigorously in test tube A. Hydrochloric acid (HCl) is a
strong acid whereas acetic acid (CH3COOH)
is a weak acid. Being strong acid, the hydrochloric acid solution contains a
much greater amount of hydrogen ions in it due to which the fizzing will occur
more vigorously in test tube A (containing hydrochloric acid). The fizzing is
due to the evolution of hydrogen gas which is formed by the action of acid on
the magnesium metal of magnesium ribbon.
Question 11:-Fresh milk has a pH of 6. How do you think the pH will
change as it turns into curd? Explain your answer.
Answer:
pH of milk falls below 6 as it turns into curd due to the formation of lactic
acid during this process. Lactic acid present in it reduces its pH value.
Question 12:-A milkman adds a very small amount of baking soda to fresh
milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
(b) Why does this milk take a long time to set as curd?
Answer:
(a) Milk is made slightly alkaline so that it may not get sour easily due to the
formation of lactic acid in it.
(b) The alkaline milk takes a longer time to set into curd because the lactic
acid being formed has to first neutralise the alkali present in it.
Question 13:-Plaster of Paris should be stored in a moisture proof
container. Explain why?
Answer:
Plaster of Paris should be stored in a moisture proof container because the
presence of moisture can cause slow setting of plaster of Paris by bringing
about its hydration. This will make the plaster of Paris useless after sometime.
Question 14:-What is a neutralisation reaction ? Give two examples.
Answer:
The reaction between an acid and a base to form salt and water is called a
neutralisation reaction.
Examples:
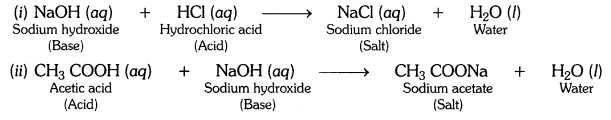
Question 15:-Give two important uses of washing soda and baking soda.
Answer:
Uses of washing soda :
(i) Washing soda is used in glass, soap and paper industries.
(ii) It is used for removing permanent hardness of water.
Uses of baking soda :
(i) Baking soda is used as an antacid in medicines to remove acidity of the
stomach.
(ii) Baking soda is used for making baking powder (used in making cakes, bread,
etc.).