Science Chapter 5 - Periodic Classification of Elements
IMPORTANT NOTES
- Early chemists classified elements as metals and
non-metals on the basis of a set of physical and chemical properties.
- Dobereiner classified elements on
the basis of “Law of Triads” which states that “atomic mass of the middle
element of a triad is almost the arithmetic mean of the atomic mass of other two
elements”.
- Newland classified elements on the
basis of “Law of Octaves” stated by him. According to this law: “When the
elements are arranged in the order of increasing atomic mass, the properties of
the eighth element (starting from a given element) are a repetition of the
properties of the first element.”
- Mendeleev’s Periodic Law states:
“The physical and chemical properties of elements are the periodic function of
their atomic masses”.
- Mendeleev’s Periodic Table is a tabular chart,
representing systematic arrangement of elements in groups and periods in the
order of their increasing atomic masses.
- In the original Mendeleev’s Periodic Table:
- There are eight vertical columns called groups. The
groups from I to VII are subdivided into two groups, i.e.,
subgroup ‘a’ and subgroup ‘b’. Thus, on the whole, there are 15 vertical
columns.
- The properties of elements in the same subgroup or main
group are similar.
- The horizontal rows in the periodic table are called
periods.
- In a period, the properties of elements gradually
change from metallic to nonmetallic character.
- There are few gaps in the periodic table. These gaps
were left knowingly as these elements were not known at that time.
- H.G.J. Moseley modified Mendeleev’s
Periodic Law by changing atomic mass to atomic number, which is a more
fundamental property of an element. It states: “The physical and chemical
properties of elements are the periodic function of their atomic number”.
- Modern Periodic Law: It states that
the properties of elements are the periodic function of their atomic numbers.
- Long form of the Periodic Table: In
the long form of the periodic table, the elements are arranged in groups and
periods on the basis of electronic configuration of elements.
- Characteristic of the Long form of the Periodic
Table.
Characteristics of the groups:
- There are
18 groups in the long form of the periodic table
- Group 1 is on the extreme left hand side and group 18 on the extreme right hand
side of the periodic table.
- Groups 1, 2 and 13 to 17 contain normal elements. The normal
elements are sometimes called representative elements. In these
elements, all the inner shells are completely filled with electrons,
except the outermost shell which is incomplete.
- The
elements in group 18 are known as noble gases or inert gases.
They have 8 electrons in their valence shell, except helium,
which has 2 electrons in the valence shell.
- The elements in group 3 to group 12 are called
transition elements. In transition elements, the
outermost shells as well as the shell next to the outermost shell
(penultimate shell) are incomplete.
Characteristics of Periods:
- There are
seven periods in all, such that each period has consecutive (or continuous)
atomic numbers.
- The
number of elements in a period corresponds to the maximum number of electrons
which can be accommodated in one shell.
- The
number of the period to which an element belongs is given by the number of the
outermost shell (quantum number).
- Rare Earth or Lanthanides: They are
inner transition elements from atomic number 57 (Lanthanium) to atomic number 71
(Lutetium). They are kept outside of the periodic table to mark their peculiar
properties.
- Actinides: They are inner transition
elements from atomic number 89 (Actinium) to atomic number 103 (Lawrencium).
They are kept outside of the periodic table to mark their peculiar properties.
- On moving from left to right in a period, the atomic
size of elements decreases in groups 1,2, 13, 14, 15, 16, 17 and then suddenly increases.
- On moving from left to right in a period, the metallic
character of elements gradually decreases and non-metallic character gradually
increases, till in the 18th group it becomes noble gas.
- All the elements in a group generally have same valency
which is equal to the number of electrons in the valence shell.
- The atomic size of elements increases as one move down
the group.
17. While moving down in the group of
metals (1, 2, 13), the metallic character of elements increases.
- While moving
down in the group of nonmetals (14, 15, 16 and 17) the electronegative character
of nonmetals decreases.
- While
moving down in the group of nonmetals (14, 15, 16 and 17) the
electronegative character of nonmetals decreases
VERY SHORT ANSWER QUESTIONS
IMPORTANT QUESTIONS
1. Who propounded Law of Triads? Give one example of
triads.
2. Why were there no zero groups in the original
Mendeleev’s Periodic Table?
3. Three elements A, B and C with similar properties
have atomic masses X, Y and Z respectively. The mass of Y is approximately equal
to the average mass of X and Z. What is such an arrangement of elements called?
Give one example of such a set of elements.
4. Elements have been arranged in the following
sequence on the basis of their increasing atomic masses. F, Na, Mg, Al, Si, P,
S, Cl, Ar, K
(a) Pick two sets of elements which have similar properties.
(b) The given sequence represents which law of classification of
elements?
5. An element with atomic number 11 is an alkali
metal. Into which families would you place elements with atomic number 10 and
12?
6. In Mendeléev’s Periodic Table the elements were
arranged in the increasing order of their atomic masses. However, cobalt with
atomic mass of 58.93 amu was placed before nickel having an atomic mass of 58.71
amu. Give reason for the same.
7. “Hydrogen occupies a unique position in the Modern
Periodic Table”. Justify the statement.
8. Write the formulae of chlorides of Eka-silicon and
Eka-aluminium, the elements predicted by Mendeleev.
9. If an element X is placed in group 14, what will be
the formula and the nature of bonding of its chloride?
10. Compare the radii of two species X and Y. Give
reasons for your answer.
(a) X has 12 protons and 12 electrons.
(b) Y has 12 protons and 10 electrons.
11. Arrange the following elements in increasing order
of their atomic radii.
(a) Li, Be, F, N
(b) Cl, At, Br, I
12. Identify the elements with the following property
and arrange them in increasing order of their reactivity.
(a) An element which is a soft and reactive metal (b) The metal which is
an important constituent of limestone
(c) The metal which exists in liquid state at room temperature.
QUESTIONS FROM CBSE EXAMINATION PAPERS
1. How and why does the atomic size vary as you go?
(i) From left to right along a period? (ii)
Down a group?
2. How will the tendency to gain electrons change as
we go from left to right across a period? Why?
3. Lithium, sodium and potassium form a Dobereiner’s
triad. The atomic masses of lithium and potassium are 7 and 39 respectively.
Predict the atomic mass of sodium.
4. Chlorine, bromine and iodine form a Dobereiner’s
triad. The atomic masses of chlorine and iodine are 35.5 and 126.9 respectively.
Predict the atomic mass of bromine.
5. Calcium, strontium and barium form a Dobereiner’s
triad. The atomic masses of calcium and barium are 40 and 137 respectively.
Predict the atomic mass of strontium.
6. State the first limitation of Mendeleev’s Periodic
Table.
7. Why was the system of classification of elements
into triads not found suitable?
8. Why could no fixed position be given to hydrogen in
Mendeleev’s Periodic Table?
9. What are ‘groups’ and ‘periods’ in the periodic
table?
10. Why did Mendeleev have gaps in his periodic table?
11. Element M forms a chloride with the formula MCl2
which is a solid with a high melting point. To which group of the periodic table
does the element ‘M’ belong?
SHORT ANSWER QUESTION
IMPORTANT QUESTIONS
1. Why was there a necessity for classification of
elements? Give at least two reasons.
2. State two achievements of Law of Octaves.
3. Can the following groups of elements be classified
as Dobereiner’s triad?
(a) Na, Si, Cl (b) Be, Mg, Ca Atomic mass of Be 9; Na
23; Mg 24; Si 28; Cl 35; Ca 40. Explain by giving reason.
4. (i) What are transition elements?
(ii) Which amongst the following are transition
elements? K, Mn, Ca, Cr, Cu Cs, Fe and Pt.
5. Give the name and symbol for the element that
occupies each of the following positions in the periodic table:
(i) Period 2, group 13
(ii) Period 1, group 1
(iii) Period 4, group 2
(iv) Period 3, group 17
6. Silicon and phosphorus (atomic no. 14 and 15,
respectively) belong to the same period of the long form of the periodic table.
Write down their electronic configurations and state in which group these
elements occur.
7. How many periods are called short periods in the
long form of the periodic table? Give their : (i) periodic numbers, (ii) number
of elements in each period, (iii) name one element in each period.
8. Amongst the elements given below, pick out the
elements which are : (i) most electropositive, (ii) most electronegative and
(iii) noble gas.
Li, Be, B, C, N, O, F, Ne, Na,
Mg, Al, Si, P, S, Cl, Ar, K and Ca.
9. How is the atomic volume of sodium related to (i)
magnesium and (ii) potassium?
10. P (186 pm), Q (231 pm), R (152 pm) P, Q and R are
the elements, such that their atomic radii is shown in brackets. Furthermore,
they have same number of electrons in their valence shell.
(i) Do these elements belong to the same group or same
period?
(ii) Arrange the elements, such that the most metallic
element comes first and the least metallic element comes last.
11. Mendeleev predicted the existence of certain
elements not known at that time and named two of them as Eka-silicon and
Ekaaluminium.
(a) Name the elements which have taken the place of
these elements.
(b) Mention the group and the period of these elements
in the Modern Periodic Table.
(c) Classify these elements as metals, nonmetals or
metalloids.
(d) How many valence electrons are present in each one
of them?
12. Identify and name the metals out of the following
elements whose electronic configurations are given below:
(a) 2, 8, 2
(b) 2, 8, 1
(c) 2, 8, 7 (d) 2, 1
QUESTIONS FROM CBSE EXAMINATION PAPERS
1. State two main properties of elements on which
Mendeleev’s periodic classification was based. Why could no fixed position be
assigned to hydrogen in his periodic table?
2. (a) What is meant by periodicity in properties of
elements with reference to the periodic table?
(b) Why do all elements of the same group have similar
properties?
3. Two elements M and N belong to groups I and II
respectively and are in the same period of the periodic table. How do the
following properties of M and N vary?
(i) Sizes of their atoms
(ii) Their metallic characters
(iii) Their valencies in forming oxides
(iv) Molecular formulae of their chlorides
4. The elements of the third period of the periodic
table are given below:
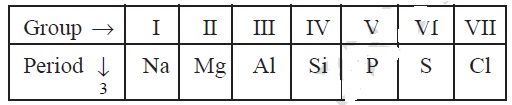
(a) Which atom is bigger, Na or Mg? Why?
(b) Identify the most (i) metallic (ii)
nonmetallic element, in period 3.
5. An element has electronic configuration 2, 8, 3.
What is the atomic number of this element?
To which (i) group and (ii) period this element
belongs?
6. State the positions of (i) isotopes of the same
element and (ii) hydrogen in the Modern Periodic Table.
7. How does the tendency to gain electrons change as
we go down the 16th group of periodic table? Why?
8. Give reasons for the following:
(a) Lithium atom is smaller than sodium atom.
(b) Chlorine (atomic Number 17) is more electronegative
than sulphur (atomic Number 16).
9. (a) State Modern Periodic Law. (b) State the place
of metalloids in the periodic table.
10. (a) State the Modern Periodic Law.
(b) Name the element which has twice as many
electrons in its second shell as in its first shell. Write its electronic
configuration also.
11. (a) What is common in the elements belonging to
the same period of periodic table?
(b) Why are chlorine and bromine kept in
the same group of the periodic table?
12. An element belongs to third period and second
group of the periodic table.
(a) State number of valence electrons in it.
(b) Is it a metal or a nonmetal?
(c) Name the element.
(d) Write the formula of its oxide.
13. Account for the following:
(a) Elements C, N, O and F are all placed in the second
period of the periodic table.
(b) Elements of group 17 are monovalent.
14. (a) How does atomic radius change as we move from
left to right in a period?
(b) The positions of three elements P, Q
and R in the periodic table are shown below
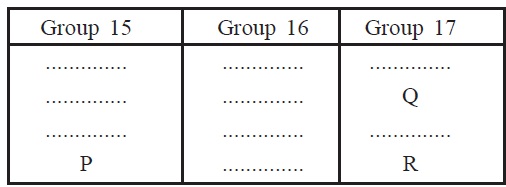
Which one of the three elements is most nonmetallic?
15. This question refers to the elements of the
periodic table with atomic numbers 3 to 18.
(a) Which of them are noble gases?
(b) Which of them are halogens?
(c) Which of them are alkali metals?
(d) What is the electronic configuration of an element
with atomic number 10?
16. Account for the following:
(a) although hydrogen resembles halogens in properties yet
it is placed in group I of the
periodic table.
(b) Elements of group-18 are called
zerovalent.
17. The position of three elements A, B and C in the
periodic table is shown below:
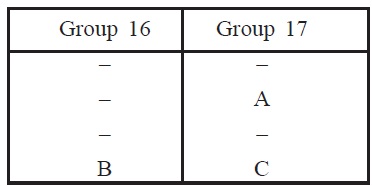
(a) State whether A is a metal or nonmetal.
(b) State whether C is more reactive or less reactive
than A.
(c) Will C is larger or smaller in size than B?
(d) Which type of ion, cation or anion, will be formed by element A?
18. (a) State Modern Periodic Law.
(b) Elements A, B, C and D have atomic
number 1, 8, 11 and 19 respectively. Choose the odd element and give reason for
your answer.
19. An element X has atomic number 19.
(a) Write its electronic configuration. (b) To which
group of the Modern Periodic Table does it belong?
(c) State the nature of the compound formed by element X with
chlorine. (d) Write the valency of element X.
20. An element X has mass number 35 and number of
neutrons 18.
(a) Write the atomic number of X. (b) Give electronic
configuration of X.
(c) To which group and period does it belong?
21. A part of the periodic table has been shown below:
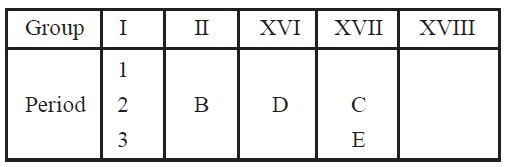
On the basis of above table answer the following questions:
(i)
Which element will form cation?
(ii) Which element will have the smallest atomic size?
(ii)
Which element will have chemical properties similar to
magnesium (atomic number 12)?
(iv) Write the common name of the group to which C and E
belong.
22. A metal ‘X’ forms an oxide having the formula XO.
It belongs to third period in the Modern Periodic Table. Write the atomic
number, valency, electronic configuration and name of the group to which the
element belongs.
23. Fluorine (atomic number 9) and chlorine (atomic
number 17) are members of the periodic table.
(i) Write their electronic configurations. (ii)
Which one is more electronegative? Give one reason.
24. Two elements ‘X’ (atomic number 7) and ‘Y’ (atomic
number 15) belong to group 15 of the periodic table. Write the electronic
configuration of these elements. Which of these will be more electronegative and
why?
25. State Mendeleev’s Periodic Law. Write to
achievements of Mendeleev’s Periodic Table.
26. The elements of the second period of the periodic
table are given below: Li Be B C N O F
(a) Give reason to explain why atomic radii
decreases from Li to F.
(b) Identify the most (i) metallic (ii)
nonmetallic element.
27. What physical and chemical properties of elements
were used by Mendeleev in creating his periodic table? List two observations
which posed a challenge to Mendeleev’s Periodic Law.
SHORT ANSWER QUESTIONS
IMPORTANT QUESTIONS
1. Lithium, sodium and potassium are put in the same
group on the basis of similar properties.
(i) What is the similarity in their properties?
(ii) If the atomic mass of lithium is 7 and
potassium is 39, calculate the atomic mass of sodium.
2. Explain, why the reducing power of an element
decreases on moving from left to right in a period of the periodic table.
3. By giving reasons, state which amongst the elements
given below does not belong to the same period:
![]()
4. Table shows three elements A, B and C along with
their electronic configuration.
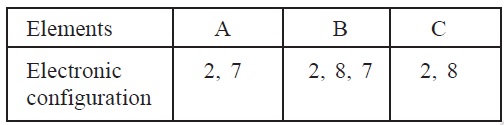
(i)
Which elements belong to same period?
(ii)
Which element belongs to group 18?
(iii)
Which elements belong to same group?
(iv)
Which element amongst A and B, is less reactive?
5. Amongst the elements P (atomic number 4), Q (atomic
number 11) and R (atomic number 20), which two elements have similar chemical
properties and why?
6. Properties of the elements are given below. Where
would you locate the following elements in the periodic table?
(a) A soft metal stored under kerosene.
(b) An element with variable (more than one) valency stored
under water.
(c) An element which is tetravalent and forms the basis of
organic chemistry.
(d) An element which is an inert gas with atomic number 2.
(e) An element whose thin oxide layer is used to make other
elements corrosion resistant by the process of “anodising”.
7. Write the formula of the product formed when
element A (atomic number 19) combines with element B (atomic number 17). Draw
its electron dot structure. What is the nature of the bond formed?
QUESTIONS FROM CBSE EXAMINATION PAPERS
1. An element X belongs to group 17 and third period
of the periodic table.
(a) Write electronic configuration of the element. What
is its valency?
(b) Predict its nature, whether it is a metal or
nonmetal.
(c) Give the formula of the compound formed when it
combines with an element Y having valency three.
2. Three elements A, B and C have atomic number 7, 8
and 9 respectively.
(a) What would be their positions in the Modern
Periodic Table (Mention group and period both)?
(b) Arrange A, B and C in the decreasing order of
their size.
(c) Which one of the three elements is most
reactive and why?
3. Given below are four elements with their atomic
numbers
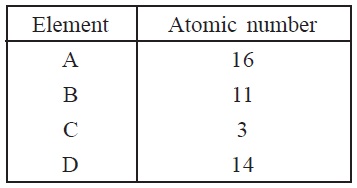
(a) Identify the elements which belong to the
same group of the Modern Periodic Table.
(b) Arrange the given elements in decreasing
order of atomic size.
(c) Write the formula of the oxide of B.
(d) Which of the above elements is a metalloid?
4. (a) The elements of the second period along with
their atomic number in parentheses are given below :
B (5), Be (4), O (8), N (7), Li (3), C (6), F (9)
(i) Arrange them in the same order as they are in the
periodic table.
(ii) Which element has the (i) largest (ii) smallest
atom?
(b) Why does the atomic radius change as we move from left to
right in a period?
5. (a) How is the valency of an element determined
from its position in the periodic table?
(b) Magnesium has atomic number 12. To which (i) group
(ii) period of the periodic table does it belong?
(c) The valency of all the elements in a group is same. Why?
6. A part of modern periodic table is given below.
Answer the following questions based on this table.
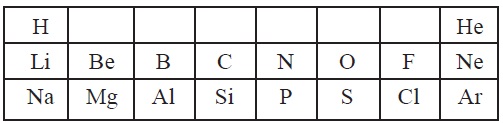
(a) Why do H, Li and Na show similar properties?
(b) Atomic size of Mg is bigger than Be, Why?
(c) Why are He, Ne and Ar called noble gases?
(d) Write a common name of the family to which F and Cl belong.
(e) Write the trend of nonmetallic character in the
horizontal row from Na to Cl.
(f) How does the atomic size vary as we move from Li to
F in the second period of periodic table?
7. The position of four elements A, B, C and D in the
periodic table are shown below.
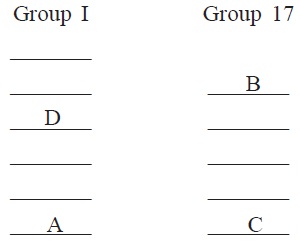
(a) Name most electronegative element.
(b) Name most reactive metal.
(c) State whether B is a metal or a nonmetal.
(d) Which one of the given elements is expected to have
largest atomic radius?
(e) How many electrons are present in the outermost shell of
elements B and C?
(f) What will be the nature of the bond formed between D and
B?
8. (a) Name an element you would expect to show
chemical reactions similar to sodium. State the reason in support of your
answer.
(b) Write electronic configuration of the element
belonging to 3rd period and 13th group of the periodic table. Predict
whether it is a metal or a nonmetal. Give reason.
9. A part of the periodic table has been shown below:
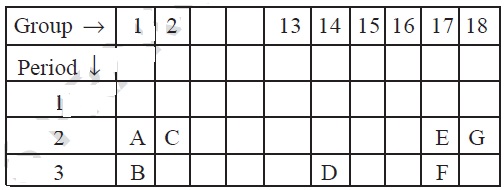
Answer the following questions on the basis of position of
elements in the above table.
(a) Which element in a noble gas? Give
reason.
(b) Which element is most electronegative?
Give reason.
(c) Write the electronic configuration of
(i) B and (ii) E.
10. Table given below shows a part of the periodic
table.
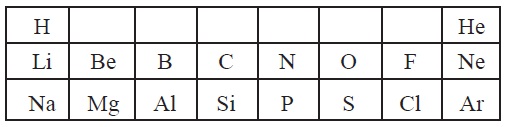
Using this table explain, why
(a) Li and Na are considered as active metals?
(b) Atomic size of Mg is less than that of Na? (c) Fluorine
is more reactive than chlorine?
11. The position of three elements A, B and C in the
periodic table are shown in table given on next page :
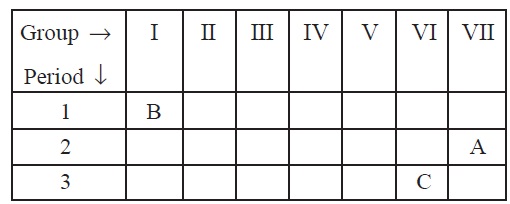
Giving reasons, explain the following:
(a) Element A is nonmetal.
(b) Atom of element C has a larger size than atom of element
A.
(c) Element B has a valency of 1.
12. State any three limitations of Mendeleev’s
classification.
13. (a) Which two criteria did Mendeleev use to
classify the elements in his periodic table?
(b) State Mendeleev’s Periodic Law.
14. The position of three elements A, B and C in the
Periodic Table are shown below:
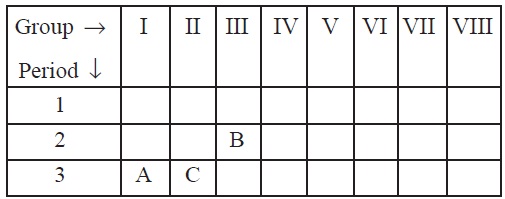
Giving reasons, explain the following:
(a) Element A is metal.
(b) Element C has a larger size than element B.
(c) Element B has a valency of 3.
LONG ANSWER QUESTIONS
IMPORTANT QUESTIONS
1. How could the Modern Periodic Table remove various
anomalies of Mendeleev’s Periodic Table?
2. Answer the following questions regarding the long
form of the periodic table.
(i) What do you understand by the term period?
How many total numbers of periods are there?
(ii) Which period is the shortest? Name the
elements in this period.
(iii) How many periods are called short
periods? Give their period numbers and name one element of each period.
(iv) How many periods are called long periods? How many
elements are there in the long periods?
(v) How many periods are called very long periods? Give
their period numbers and state which amongst them is complete.
How many elements are there in the very long complete period?
3. Answer the following questions regarding element
15 X31:
(i) What is its electronic configuration?
(ii) To which group does it belong?
(iii) To which period does it belong?
(iv) How many electrons are there in its valence
shell?
(v) What is its valency?
(vi) Is it a metal or a nonmetal?
(vii) What is the formula of its compound with
sodium?
4. (a) Electropositive nature of the elements
increases down the group and decreases across the period
(b) Electronegativity of the elements decreases down
the group and increases across the period
(c) Atomic size increases down the group and decreases
across a period (left to right).
(d) Metallic character increases down the group and decreases
across a period.
On the basis of the above trends of the periodic table, answer the
following about the elements with atomic numbers 3 to 9.
(a) Name the most electropositive element among them.
(b) Name the most electronegative element.
(c) Name the element with smallest atomic size.
(d) Name the element which is a metalloid.
(e) Name the element which shows maximum valency.
5. An element X which is a yellow solid at room
temperature shows catenation and allotropy. X forms two oxides which are also
formed during the thermal decomposition of ferrous sulphate crystals and are the
major air pollutants.
(a) Identify the element X.
(b) Write the electronic configuration of X.
(c) Write the balanced chemical equation for the
thermal decomposition of ferrous sulphate crystals.
(d) What would be the nature (acidic/ basic) of oxides
formed?
(e) Locate the position of the element in the Modern
Periodic Table.
6. An element X of group 15 exists as a diatomic
molecule and combines with hydrogen at 773 K in the presence of a catalyst to
form a compound, ammonia, which has a characteristic pungent smell.
(a) Identify the element X. How many valence electrons does
it have?
(b) Draw the electron dot structure of the diatomic molecule
of X. What type of bond is formed in it?
(c) Draw the electron dot structure for ammonia and
what type of bond is formed in it?
7. An element placed in 2nd group and 3rd
period of the periodic table, burns in the presence of oxygen to form a basic
oxide.
(a) Identify the element.
(b) Write the electronic configuration.
(c) Write a balanced equation when it burns in the
presence of air.
(d) Write a balanced equation when this oxide is
dissolved in water.
(e) Draw the electron dot structure for the formation
of this oxide.
8. Atomic number of a few elements are given as 10,
20, 7, 14.
(a) Identify the elements.
(b) Identify the group number of these elements in the
periodic table.
(c) Identify the periods of these elements in the
periodic table.
(d) What would be the electronic configuration of each
of these elements? (e) Determine the valency of these elements.
QUESTIONS FROM CBSE EXAMINATION PAPERS
1. (a) Why do we classify elements?
(b) What were the two criteria used by Mendeleev in
creating his periodic table?
(c) Why did Mendeleev leave some gaps in his periodic
table?
(d) In Mendeleev’s Periodic Table, why there was no
mention of noble gases like helium, neon and argon?
(e) Would you place the two isotopes of chlorine, Cl-35
and Cl-37 in different slots because of their different atomic masses or in the
same slot because their chemical properties are the same? Justify your answer.
2. An element X (atomic number 17) reacts with an
element Y (atomic number 20) to form a compound.
(a) In which group and period of modern periodic table the
elements X and Y are present?
(b) Classify X and Y as metal(s), nonmetal(s) or
metalloid(s).
(c) What will be the formula of oxide of element Y? Identify
the nature of the bond between the two elements in the oxide formed.
(d) Draw the electron dot structure of the cation and
anion formed when X reacts with Y.
3. Atoms of seven elements A, B, C, D, E, F and G have
different number of electronic shells but have the same number of electrons in
their outermost shells. The elements A and C combine with chlorine to form an
acid and common salt respectively. The oxide of element A is a liquid at room
temperature and is a neutral substance, while the oxides of the remaining six
elements are basic in nature. Based on the above information answer the
following questions.
(i) What could the element A be?
(ii) Will elements A to G belong to the same period or same group
of the periodic table?
(iii) Write the formula of the compound formed by the reaction of
element A with oxygen.
(iv) Show the formation of the compound by a combination of element
C with chlorine with the help of an electronic structure.
(v) What would be the ratio of the number of combining atoms in a
compound formed by the combination of element A with carbon?
(vi) Which one of the given elements is likely to have the smallest
atomic radius?
4. On the basis of Mendeleev’s Periodic Table given
below, answer the questions that follow.
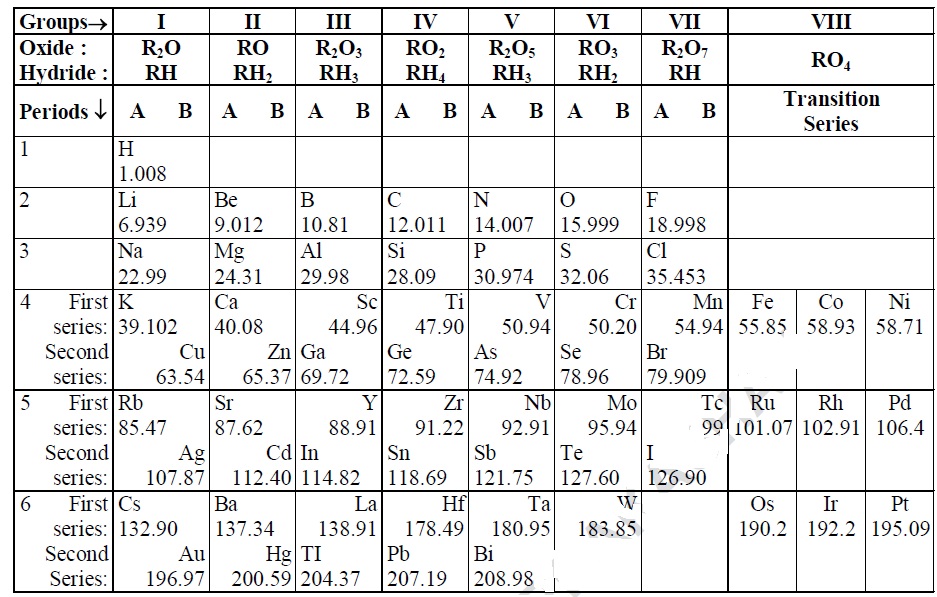
(a) Name the element which is in : (i) group I and 3rd
period. (ii) group VII and 2nd period.
(b) Suggest the formula of the following: (i)
oxide of nitrogen (ii) hydride of oxygen.
(c) In group VIII of the periodic table, why does
cobalt with atomic mass 58.93 appear before nickel having atomic mass 58.71?
(d) Besides gallium, which two other elements have since been
discovered for which Mendeleev had left gaps in his periodic table?
(e) Using atomic masses of Li, Na and K, find the
average atomic mass of Li and K, and compare it with the atomic mass of
Na. State the conclusion drawn from this activity.