Science Chapter 3 - Metals and Non-metals
IMPORTANT NOTES
- Element is a substance which cannot
be further subdivided into simpler substances by any physical or chemical means.
- Metals are the elements (except
hydrogen) which form positively charged ions by losing electrons from their
valence shell and form oxides which are basic in nature.
- Non-metals are the elements which
form negatively charged ions by accepting electrons in their valence shell and
form acidic or neutral oxides.
- All metals have one to three
electrons in their valence shell.
- All non-metals have four to seven
electrons in their valence shell.
- Physical Properties of Metals Metals
generally :
(i) are solids, (ii) are hard,
(iii) have lustre,
(iv) have high densities,
(v) have high melting
and boiling points,
(vi) are malleable,
(vii) are ductile,
(viii) have high tensile
strength, (ix) are good conductors of heat and electricity,
(x) are monoatomic,
(xi) and can form alloys.
- Physical Properties of Non-metals
Non-metals generally : (i) are brittle solids or gases, (ii) are soft,
(iii) have low densities, (iv) have no lustre, (v) have low melting and
boiling points, (vi) are not malleable, (vii) are not ductile, (viii)
have no tensile strength, (ix) are bad conductors of heat and
electricity, (x) are polyatomic, (xi) do not form alloys.
- Chemical Properties of Metals :
(i) Metals generally
react with oxygen to form their oxides which are basic in nature.
(ii) Metal oxides of
aluminium, zinc, lead and tin react with alkalises as well as acids. Such oxides
are called amphoteric oxides.
(iii) Active metals like potassium,
sodium, calcium, magnesium, aluminium, zinc and iron react with water or steam
to form their hydroxides/oxides and hydrogen gas.
(iv) Active metals react with
dilute mineral acids to form their respective salts and hydrogen gas.
(v) Active metals displace less
active metals from their aqueous salt solutions. The reaction which takes place
is called chemical displacement reaction.
(vi) A table of metals arranged in the order of
their decreasing chemical reactivity, is called metal reactivity series.
- Chemical Properties of Non-metals
(i) Non-metals generally react with oxygen to
form their oxides, which are either neutral or acidic in nature.
(ii) Neutral oxides of non-metals are CO, NO, H2O and N2O.
(iii) Non-metals do not displace hydrogen from water or dilute mineral
acids.
(iv) Non-metals react with one another to form covalent compounds.
(v) Non-metals react with metals to form ionic compounds.
- An atom or an ion having duplet or octet configuration
like noble gases is said to be in the minimum state of energy and
hence is chemically inactive.
- The atoms of an element can attain stable electronic
configuration of the nearest noble gas :
- by donating (losing) one or more electrons from their
valence shell to another atom,
- by accepting (gaining) one or more electrons in their
valence shell from another atom,
- by sharing electrons from their valence shell with
another atom/atoms.
- The atom which accepts or donates electron/ electrons
from its valence shell so as to acquire a configuration of the nearest noble gas
gets electrically charged and becomes an ion.
- The metals generally donate
electrons from their valence shell and hence form positively
charged ions. These positively charged ions are called
cations, because, they discharge at the cathode to form
neutral atoms.
- The non-metals generally accept
electrons in their valence shell and hence form negatively
charged ions. The negatively charged ions are called anions,
because, they discharge at the anode to form neutral atoms.
- Characteristics of Cations :
- Only metals form cations, because, they have 1 to 3
electrons in their valence shell which they can easily donate to acquire a
stable configuration of the nearest noble gas.
- There is no change in atomic number of an element as it
forms a cation, because, the number of protons do not change.
- The atomic radii of a cation is smaller than neutral
atom, because of the disappearance of the valence shell.
- Characteristics of Anions :
- Only non-metals form anions, because, they have 4 to 7
electrons in their valence shell. Thus, they accept electrons in their valence
shell to acquire a stable configuration of the nearest noble gas.
- There is no change in the atomic number of an anion as
the number of protons in it are the same as in the neutral atom.
- The atomic radii of an anion slightly increases, because
the effective pull of the nucleus slightly decreases due to addition of extra
electron/electrons in the valence shell.
-
Electropositive
elements: The elements which have a tendency to donate
electrons from their valence shell and become positively charged ions (cations)
are called electropositive elements. All metals and hydrogen are
electropositive elements.
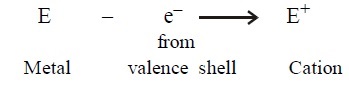
- Electronegative elements: The
elements which have a tendency to accept electrons in their valence shell and
become negatively charged ions (anions) are called electronegative
elements. All non-metals are electronegative elements.

- Electrovalent bond or Ionic bond: A
chemical bond formed between two different atoms, by the transfer of one or more
electrons from the valence shell of an electropositive or metallic element to
the valence shell of a nonmetallic element, is called an electrovalent
bond or an ionic bond.
- Electrovalency : The number of
electrons which an atom of an element donates or accepts in its valence shell,
so as to have a stable configuration like that of the nearest noble gas is
called electrovalency.
- Electropositive valency : The number
of electrons which an atom of an element (metal or hydrogen) donates from its
valence shell, so as to have a stable configuration like that of a noble gas, is
called electropositive valency.
- Electronegative valency : The number
of electrons which an atom of an element (nonmetal) accepts in its valence
shell, so as to have a stable configuration like that of a noble gas is called
electronegative valency.
- Electrovalent compound or Ionic compound:
The chemical compound formed as a result of transfer of electrons
from the valence shell of an atom (metal or hydrogen) of an element to
the valence shell of an atom of another element (non-metal) is
called electrovalent compound or ionic compound.
- Properties of Electrovalent (ionic) Compounds:
- They are generally hard and crystalline solids.
- They are generally non-volatile and hence have high
melting and boiling points.
- They are good conductors of electricity in the fused
state.
- They are generally soluble in water and their aqueous
solutions are good conductors of electricity.
- The chemical reaction between the aqueous solutions of
ionic compounds is very fast.
- Metallurgy encompasses various
processes in the extraction of a metal from its ore and then refining the metal
including study of its properties and uses.
- Gangue or Matrix are the unwanted
impurities, such as sand, stones, mud, limestone, mica, etc. associated with the
naturally occurring ore.
- Dressing of ore involves processes,
(such as hand picking, grinding and crushing and pulverizing) which give an ore
such a physical form, so that gangue can be easily removed from the ore.
- Concentration of ore involves
processes, which help in the removal of gangue from the dressed ore, thereby
increasing the concentration of the metal in the ore.
- Electromagnetic separation is the
concentration process followed for the dressed ore, if
- the ore is magnetic in nature.
- ore contains magnetic impurities (such as Fe2O3).
- Gravity process or Hydraulic washing method
of concentration is followed for such dressed ores which have
metallic ores of high density as compared to the density of gangue. It
is not followed in case of sulphide ores.
- Froth floatation process for the
concentration is followed for sulphide ores only. In this process, the sulphide
ore is immersed in a mixture of pine oil and water and then strongly agitated
with compressed air. The sulphide ore rises up along with the froth produced by
the
oil, but the gangue sinks to the bottom.
- Chemical method for the concentration of ore
is followed for such ores (ore of aluminium), in which density
of the ore and the gangue is almost same.
- Calcination is the process of
heating the concentrated ore in the absence of air, such that it decomposes to
form its metallic oxide. Following are the objectives achieved during
calcination:
- removes moisture from the ore
- makes the ore porous
- expels the volatile impurities
- decomposes carbonate ores to oxide ores.
- Roasting is the process of heating
the concentrated ore (only sulphide ores) in the presence of excess of air, such
that it changes to the oxide ore.
Following are the objectives achieved during roasting:
- removes moisture from the ore
- makes the ore porous
- expels the volatile impurities
- oxidises sulphide ores to oxide ores.
- Smelting or reduction of ore is the
process of conversion of the metal oxide ore into metal, by reducing it with a
suitable reducing agent. The reducing agents commonly used are coke, carbon
monoxide and hydrogen. For reducing ores of highly active metals, electrolytic
reduction is employed.
- Refining of metals is done by a
number of methods. However, the best method is electrolytic method. In this
method the pure metal is made the cathode, and the impure metal is made the
anode. The cathode and the anode are immersed in the aqueous solution of metal.
On the passage of electric current, the pure metal from the anode is transferred
to the cathode.
- Thermite mixture consists of three
parts of ferric oxide and one part of powdered aluminium. It is commonly
employed in spot welding, such as broken railway lines.
- Alloy is a homogeneous mixture of
two ormore metals, obtained by melting them together.
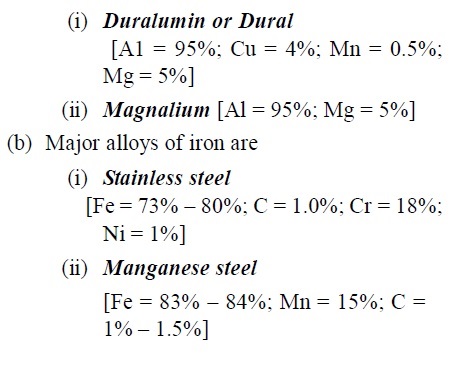
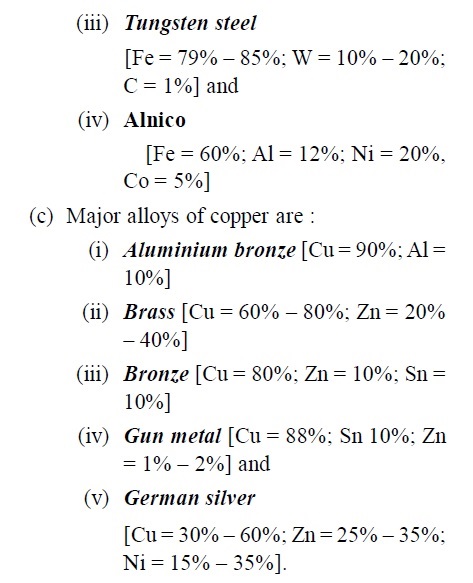
- (a) Major alloys of aluminium are
- Gold is alloyed with metals like
copper, silver, cadmium, so as to make it hard and workable at low temperature.
- Purity of gold is measured in
carats. 100% pure gold is 24 carat, while 1 carat = 4.1666 g per 100 g of alloy.
- Corrosion of metals is the formation
of layers of undesirable compounds on the surface of metals due to the action of
moist air containing impurities.
- Corrosion of metal take place only,
if the surface of metal comes in direct contact with moist air for prolonged
time.
- Rusting: The slow conversion of iron
into hydrated ferric oxide in the presence of moist air is called rusting.
- Rust is a flaky, non-sticky brown
powder formed on the surface of iron, when the iron is exposed to moist air.
- Factors which promote rusting : In
addition to moist air : (i) the presence of salts such as sodium chloride, (ii)
presence of more active metals than iron and the presence of pollutants such as
NO2; SO2; CO2 in air, promote rusting.
- Rusting can be prevented by coating
the metal surface with (i) red lead (ii) paints
(iii) enamel (iv) oil or grease (v) plastic coating (vi) galvanising (vii) tinning (viii) electroplating with nickel or chromium (ix) converting iron into stainless steel.
VERY SHORT ANSWER QUESTIONS
IMPORTANT QUESTIONS
1. Name two acidic non-metallic oxides.
2. Name a non-metal which is highly tensile.
3. Name a non-metal which forms positively charged
ions.
4. Name two metals whose density is less than 1 gcm–3.
5. Name a naturally occurring non-metal, which is the
hardest substance.
6. Name a metal which does not react with conc. nitric
acid.
7. Generally, when metals are treated with mineral
acids, hydrogen gas is liberated but when metals (except Mn and Mg) are treated
with HNO3, hydrogen is not liberated, why? [HOTS]
8. Amongst the metals, non-metals and noble gases, to
which category do the element belong if they have:
(i) positive
valency; (ii) negative valency; (iii) zero valency.
QUESTIONS FROM CBSE EXAMINATION PAPERS
1. Why do silver ornaments lose their shine when kept
for some time?
2. Name a metal other than aluminium that is covered
with an oxide film layer.
3. Name one metal and one non-metal which exists in
liquid state at room temperature?
4. Name a non-metal which is lustrous and a metal
which is non-lustrous.
5. Name two metal which have very low melting point.
6. If copper metal is heated over a flame, it develops
a coating. What is the colour and composition of this coating?
7. Why is sodium metal kept immersed in kerosene oil?
8. Name one metal which react with very dilute HNO3
to evolve hydrogen gas.
9. A non-metal X exists in two different forms Y and
Z. Y is the hardest natural substance, whereas Z is a good conductor of
electricity. Identify X, Y, and Z.
10. An element A form two oxides AO and AO2.
The oxide AO is neutral whereas the oxide AO2 is acidic in nature.
Would you call element A a metal or non-metal.
11. In the refining of silver the recovery of silver
from silver nitrate solution involves displacement by copper metal. Give the
reason for the same.
12. Name two metals which are both ductile as well as
malleable.
13. The reaction of iron (III) oxide Fe2O3
with aluminium is used to join cracked iron parts of machines.
14. Give reason for the following:
(a) Ionic compounds conduct electricity in the
molten state.
15. Give reason for the following: Metals can be given
different shapes according to our needs.
16. How will you test for the gas which is liberated
when hydrochloric acid reacts with an active metal?
17. Which reducing agent is used in the reduction of
alumina?
18. What are metalloids?
19. Why are titanium and chromium classified as
strategic metals?
20. Which one of the following metals does not react
with oxygen even at high temperatures?
(i) Calcium (ii)
Gold (iii) Sodium
21. Give reasons for the following: Addition of some
silver to pure gold for making ornaments.
22. Give reason for the following: Alumina is
dissolved in molten cryolite for electrolysis to obtain aluminum metal.
23. Write the chemical equation to represent the
reaction taking place between sodium metal and cold water.
24. Why is tungsten metal selected for making
filaments of incandescent lamp bulbs?
25. Name a metal which offer higher resistance to the
passage of electricity than copper.
26. Write the chemical equation for the reaction of
hot aluminium with steam.
27. How does the metal magnesium differ from the metal
calcium in their reaction with water?
28. What is seen to happen when a piece of sodium
metal is dropped into water?
SHORT ANSWER QUESTIONS
IMPORTANT QUESTIONS
1. Describe briefly the froth floatation process for
the concentration of sulphide ores.
2. What is a thermite reaction?
3. Does every mineral have a definite and fixed
composition? Explain.
4. What important properties of aluminium are
responsible for its great demand in the industry?
5. Why is iron more useful when it is mixed with a
little carbon?
6. What is 24 carat gold? How will you convert it into
18 carat gold?
7. Name the alloy of:
(i) aluminium in the construction of aircrafts,
(ii) lead used in joining metal for electrical
work,
(iii) copper used in household vessels.
8. Zinc is higher in the electrochemical series than
iron, yet it is used for preventing the rusting of iron. Explain.
9. Give the reaction involved during extraction of
zinc from its ore by
(a) roasting of zinc ore
(b) calcination of zinc ore
10. Explain the following:
(i) Iron articles are galvanised.
(ii) Metals like Na, K, Ca and Mg are never found in
their free state in nature.
11. What happen when?
(a) ZnCO3 is heated in the absence of oxygen?
(b) a mixture of Cu2O and Cu2S is
heated?
12. What are the constituents of solder alloy? Which
property of solder makes it suitable for welding electrical wires?
13. Name two metals which displace hydrogen from
strong caustic alkalises. Write chemical equations in support of your answer.
14. Name two non-metals which occur in:
(i) solid state,
(ii) gaseous state.
15. What kind of oxide is ZnO? Support your answer by
writing two chemical equations.
16. Name one metal which:
(i) displaces copper,
(ii) does not displace copper, from copper
nitrate solution.
17. What happens when iron is placed in copper
sulphate solution?
18. State the reactions if any of the following metals
reacts with ferrous sulphate solution:
(i) zinc, (ii) copper,
(iii) silver.
19. A metal P is placed in an aqueous solution of Q.
In a few hours metal Q was deposited on metal P. Which metal amongst P and Q is
more reactive and why?
20. Write chemical equations for the following
reactions.
(i) Aluminium and hydrochloric acid
(ii) Magnesium and steam
QUESTIONS FROM CBSE EXAMINATION PAPERS
1. Aluminium occurs in combined state whereas gold is
found in Free State. Why?
2. Most metals do not react with bases, but zinc metal
does. Suggest a reason. Write an equation for the reaction between Zn and NaOH.
3. Write chemical equations for the reactions taking
place when
(i) zinc sulphide is heated in air
(ii) calcination of zinc carbonate is done.
4. How pure copper is obtained from impure copper by
electrolytic refining?
5. When a metal X is treated with cold water, it gives
a basic salt Y with molecular formula XOH (Molecular mass = 40) and liberates a
gas Z which easily catches fire. Identify X,Y,Z.
6. Write the equations for the following metals which
are obtained from their compounds by reduction process.
(i) Metal X which is low in reactivity series.
(ii) Metal Y which is middle of series.
7. Explain, why most of the metals do not displace
hydrogen from Nitric acid.
8. Explain, why calcium metal after reacting with
water starts floating on its surface? Write thechemical equation for the
reaction.
9. Name the chemicals used in the acid fire
extinguisher and the gas evolved from it when used?
10. State reasons for the following :
(i) Electric wires are covered with rubberlike
material.
(ii) From dilute hydrochloric acid zinc can liberate
hydrogen gas but copper cannot.
11. State reasons for the following observations:
(i) The shining surface of some metals becomes
dull when exposed to air for a long time.
(ii) Metals sulphides occur mainly in rocks but metal
halides occur mostly in sea and lake.
12. Differentiate between roasting and calcination
processes used in metallurgy. Give an example of each.
13. Give reason for the following:
(a) Gold and silver are used to make jewellery.
(b) Carbonate and sulphide ores are usually converted
into oxides prior to reduction during the process of extraction.
14. With a labelled diagram describe an activity to
show that metals are good conductors of electricity.
15. Name an alloy
(i) Which has a lower melting point than its constituents.
(ii) Which is more hard, tough and strong than its
constituents.
16. Define the term ‘alloy’. Write two advantages of
making alloys.
17. State reasons for the following:
(i) Metals are good conductors of heat
(ii) Inability of non-metals for displacing hydrogen
from dilute sulphuric acid.
18. Choose the metal (from the list given below) which
can displace zinc from zinc sulphate solution-Lead, Copper, Magnesium, Silver.
Write the equation of the chemical reaction involved.
19. A copper plate was dipped into a solution of
AgNO3. After sometime, a black layer was deposited on the copper plate. State
the reason for it. Write the chemical equation of the reaction involved.
SHORT ANSWER QUESTIONS
IMPORTANT QUESTIONS
1. Iqbal treated a lustrous, divalent element M with
sodium hydroxide. He observed the formation of bubbles in the reaction mixture.
He made the same observations when this nelement was treated with hydrochloric
acid. Suggest how he can identify the produced gas. Write chemical equations for
both the reactions.
2. An alkali metal A gives a compound B (molecular
mass = 40) on reacting with water. The compound B gives a soluble compound C on
treatment with aluminium oxide. Identify A, B and C and give the reaction
involved.
3. Give one example of an article made from iron which
is protected from rusting by:
(i) red lead
paint (ii)
enamelling
(iii) plastic
coating (iv) tinning
(v)
electroplating (vi) oiling or greasing
4. During extraction of metals, electrolytic refining
is used to obtain pure metals.
(a) Which material will be used as anode and cathode
for refining of silver metal by this process?
(b) Suggest a suitable electrolyte also.
(c) In this electrolytic cell, where do we get pure
silver after passing electric current?
5. Compound X and aluminium are used to join railway
tracks.
(a) Identity the compound X
(b) Name the reaction
(c) Write down its reaction.
6. Give the steps involved in the extraction of metals
of low and medium reactivity from their respective sulphide ores.
7. (i) Why is gold alloyed? Give two reasons.
(ii) Name two metals which are commonly used for
alloying gold.
QUESTIONS FROM CBSE EXAMINATION PAPERS
1. Most metals do not react with bases but zinc metal
does. Suggest a reason and write an equation for the reaction between zinc and
NaOH.
2. A magnesium ribbon is burnt in oxygen to give a
white compound X accompanied by emission of light.
(a) Write the chemical formulae of X
(b) Write a balanced chemical equation, when X is
dissolved in water.
3. Metal compound A reacts with dilute hydrochloric
acid and to produce effervescence. The gas evolved extinguishers a burning
candle and turns the limewater milky.
Write balanced
chemical equations for the reactions.
4. (a) Why metals are not found in their free state
generally?
(b) If a strip of aluminium with scratched clean
surface is dipped into an aqueous solution of copper sulphate for little time,
surface of the strip becomes brownish. What is the reason for this? Write the
balanced chemical equation for the reaction.
5. (a) What type of reaction is to be performed to
ascertain and verify the position of metals in the reactivity series?
(b) If an iron nail immersed in the aqueous solution of
copper sulphate, what are the changes happening to the nail and to the solution?
(c) Write the balanced chemical equation for the reaction between
iron metal and aqueous copper sulphate solution.
6. (a) Using a simple experiment, how can you prove
that magnesium is placed above zinc in reactivity series?
(b) Why copper metal cannot liberate hydrogen when
reacting with dil. HCl?
7. Give reasons for the following:
(i) Zinc oxide is considered as an amphoteric oxide.
(ii) Non-metals in general do not displace hydrogen
from dilute acids.
(iii) Metals conduct electricity.
8. An ore on heating in air produces sulphur dioxide.
Which process would you suggest for its concentration? Describe briefly any two
steps involved in the conversion of this concentrated ore into the related
metal.
9. What is meant by ‘rusting’? With labelled diagrams
describe an activity to find out the conditions under which iron rusts.
10. Give reasons for the following observations:
(i) Ionic compounds in general have high melting and boiling
points
(ii) Highly reactive metals cannot be obtained from their
oxides by heating them with carbon.
(iii) Copper vessels get a green coat when left exposed to
air in the rainy season.
11. Name two metals which react violently with cold
water. Write any three observations you would make when such a metal is dropped
into water. How would you identify the gas evolved, if any, during the reaction?
12. (a) Give an example of a metal which
(i) can be easily cut with a knife
(ii) is a liquid at room temperature.
(b) Write chemical equation for the reaction when
(i) steam acts on red hot iron
(ii) zinc is added to iron (II) sulphate
solution.
13. (a) Name a metal for each case :
(i) It does not react with cold as well as hot
water but reacts with steam.
(ii) It does not react with any physical state of
water.
(b) When calcium metal is added to water the gas
evolved does not catch fire but the same gas evolved on adding sodium metal to
water catches fire. Why is it so?
14. (a) Name the chief ore of iron. Write its formula.
(b) How is an iron ore concentrated? Describe it
briefly.
15. Give reasons for the following:
(i) Metals are regarded as electropositive elements.
(ii) When a piece of copper metal is added to a solution of zinc
sulphate no change takes place, but the blue colour of copper sulphate fades
away when a piece of zinc is placed in its solution.
(iii) Articles made of aluminium do not corrode even though
aluminium is an active metal.
16. (i) Explain the term ‘roasting’ as used in
metallurgical processes. Give one suitable example for it.
(ii) What changes take place when cinnabar (HgS) is heated in
air for a long enough time?
17. Explain the following terms by giving one example
of each: (i) Mineral (ii) Ore (iii) Gangue
18. Give reasons for each of the following:
(i) Germanium is called a metalloid.
(ii) Zirconium is known as a strategic metal.
(iii) Nitrogen is used to preserve food.